New combination chemotherapy of cisplatin with an electron-donating compound for treatment of multiple cancers
- PMID: 33436996
- PMCID: PMC7804005
- DOI: 10.1038/s41598-020-80876-z
New combination chemotherapy of cisplatin with an electron-donating compound for treatment of multiple cancers
Abstract
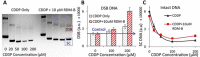
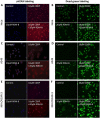
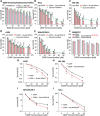
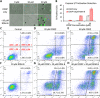
Cisplatin is the first and most widely used platinum-based chemotherapy drug and is the cornerstone agent in treating a broad spectrum of cancers. However, its clinical application is often limited by severe toxic side effects and drug resistance. Based on the discovered dissociative electron transfer mechanism of cisplatin, a novel combination of cisplatin with [9-(2-carboxyphenyl)-6-diethylamino-3-xanthenylidene]-diethylammonium chloride (basic violet 10, BV10) is proposed to potentiate the chemotherapeutic effect of cisplatin. Here, we show that this combination enhances the anti-cancer effect of cisplatin in both in vitro cell lines and in vivo xenograft mouse models of cisplatin-sensitive and -resistant lung, ovarian and cervical cancers while introducing minimal additional toxic side effects. Furthermore, femtosecond time-resolved laser spectroscopic measurements demonstrate that cisplatin reacts with BV10 via an electron transfer mechanism. These results indicate that the combination of cisplatin with BV10 is promising for improving the chemotherapy of cancers with various extents of cisplatin resistance.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Electron transfer-based combination therapy of cisplatin with tetramethyl-p-phenylenediamine for ovarian, cervical, and lung cancers.Proc Natl Acad Sci U S A. 2012 Jun 26;109(26):10175-80. doi: 10.1073/pnas.1203451109. Epub 2012 Jun 8. Proc Natl Acad Sci U S A. 2012. PMID: 22685209 Free PMC article.
-
Costunolide induces apoptosis in platinum-resistant human ovarian cancer cells by generating reactive oxygen species.Gynecol Oncol. 2011 Dec;123(3):588-96. doi: 10.1016/j.ygyno.2011.08.031. Epub 2011 Sep 25. Gynecol Oncol. 2011. PMID: 21945308
-
LY2109761 enhances cisplatin antitumor activity in ovarian cancer cells.Int J Clin Exp Pathol. 2015 May 1;8(5):4923-32. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26191185 Free PMC article.
-
Noscapine Increases the Sensitivity of Drug-Resistant Ovarian Cancer Cell Line SKOV3/DDP to Cisplatin by Regulating Cell Cycle and Activating Apoptotic Pathways.Cell Biochem Biophys. 2015 May;72(1):203-13. doi: 10.1007/s12013-014-0438-y. Cell Biochem Biophys. 2015. PMID: 25510462
-
Therapeutic potential of marine peptides in cervical and ovarian cancers.Mol Cell Biochem. 2022 Feb;477(2):605-619. doi: 10.1007/s11010-021-04306-y. Epub 2021 Dec 2. Mol Cell Biochem. 2022. PMID: 34855045 Review.
Cited by
-
Cisplatin Mouse Models: Treatment, Toxicity and Translatability.Biomedicines. 2021 Oct 7;9(10):1406. doi: 10.3390/biomedicines9101406. Biomedicines. 2021. PMID: 34680523 Free PMC article. Review.
-
Fish Collagen Peptides Protect against Cisplatin-Induced Cytotoxicity and Oxidative Injury by Inhibiting MAPK Signaling Pathways in Mouse Thymic Epithelial Cells.Mar Drugs. 2022 Mar 28;20(4):232. doi: 10.3390/md20040232. Mar Drugs. 2022. PMID: 35447905 Free PMC article.
-
Difluoromethylornithine Induces Apoptosis through Regulation of AP-1 Signaling via JNK Phosphorylation in Epithelial Ovarian Cancer.Int J Mol Sci. 2021 Sep 23;22(19):10255. doi: 10.3390/ijms221910255. Int J Mol Sci. 2021. PMID: 34638596 Free PMC article.
-
Disparities in Cisplatin-Induced Cytotoxicity-A Meta-Analysis of Selected Cancer Cell Lines.Molecules. 2023 Jul 30;28(15):5761. doi: 10.3390/molecules28155761. Molecules. 2023. PMID: 37570731 Free PMC article. Review.
-
Development of a Prostate-Specific Antigen Targeted Dual Drug Conjugate for Prostate Cancer Therapy.ACS Omega. 2025 Apr 25;10(17):17611-17625. doi: 10.1021/acsomega.4c11483. eCollection 2025 May 6. ACS Omega. 2025. PMID: 40352517 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

