Pharmacological inactivation of the prion protein by targeting a folding intermediate
- PMID: 33437023
- PMCID: PMC7804251
- DOI: 10.1038/s42003-020-01585-x
Pharmacological inactivation of the prion protein by targeting a folding intermediate
Abstract
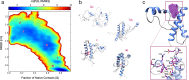
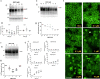
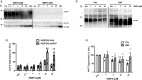
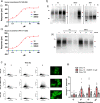
Recent computational advancements in the simulation of biochemical processes allow investigating the mechanisms involved in protein regulation with realistic physics-based models, at an atomistic level of resolution. These techniques allowed us to design a drug discovery approach, named Pharmacological Protein Inactivation by Folding Intermediate Targeting (PPI-FIT), based on the rationale of negatively regulating protein levels by targeting folding intermediates. Here, PPI-FIT was tested for the first time on the cellular prion protein (PrP), a cell surface glycoprotein playing a key role in fatal and transmissible neurodegenerative pathologies known as prion diseases. We predicted the all-atom structure of an intermediate appearing along the folding pathway of PrP and identified four different small molecule ligands for this conformer, all capable of selectively lowering the load of the protein by promoting its degradation. Our data support the notion that the level of target proteins could be modulated by acting on their folding pathways, implying a previously unappreciated role for folding intermediates in the biological regulation of protein expression.
Conflict of interest statement
The authors declare the following competing interests: G.S., G.L., L.M.B., P.F., and E.B. are co-founders and shareholders of Sibylla Biotech SRL. The company exploits the PPI-FIT technology for drug discovery in a wide variety of human pathologies, with the exception of prion diseases.
Figures



References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

