Decreased expression of adenosine receptor 2B confers cardiac protection against ischemia via restoring autophagic flux
- PMID: 33437375
- PMCID: PMC7791490
Decreased expression of adenosine receptor 2B confers cardiac protection against ischemia via restoring autophagic flux
Abstract
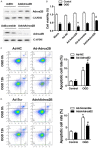
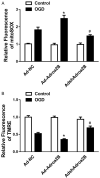
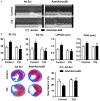
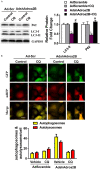
Adora2B (adenosine receptor 2B) has been reported as one of the key modulators during cardiac remodeling after acute myocardial infarction (AMI). However, the molecular mechanism involved has not been well investigated. Thus, our study aims to investigate whether Adora2B contributes to cardiac remodeling after AMI and its underlying mechanisms. Adenovirus harboring Adora2B or shAdora2B was injected in the border zone in a mouse model of AMI experimentally produced by permanent ligation of left anterior descending (LAD) coronary artery. Decreased Adora2B expression protected the cardiomyocytes from MI-induced autophagic flux obstacle, improved cardiac function, and reduced fibrosis after MI. Adora2B downregulation attenuated the accumulation of LC3-II and p62, which are autophagy substrate proteins. An adenovirus containing mRFP-GFP-LC3 showed that decreased expression of Adora2B restored the autophagic flux by enhancing autophagosome conversion to autophagolysosome. Also, Adora2B knockdown improved cardiomyocytes' survival and protected mitochondrial function of cardiomyocytes insulted with hypoxia. Notably, the effect of Adora2B on autophagy flux and cardiomyocyte protection could be mitigated by autophagy inhibitor chloroquine. Our results demonstrate that decreased expression of Adora2B protected cardiomyocytes from impaired autophagy flux induced by MI. Modulation Adora2B expression plays a significant role in blunting the worsening of heart function and reducing scar formation, suggesting therapeutic potential by targeting Adora2B in AMI for the infarct healing.
Keywords: Acute myocardial infarction; Adora2B; apoptosis; autophagy flux.
AJTR Copyright © 2020.
Conflict of interest statement
None.
Figures


References
-
- Ruparelia N, Godec J, Lee R, Chai JT, Dall’Armellina E, McAndrew D, Digby JE, Forfar JC, Prendergast BD, Kharbanda RK, Banning AP, Neubauer S, Lygate CA, Channon KM, Haining NW, Choudhury RP. Acute myocardial infarction activates distinct inflammation and proliferation pathways in circulating monocytes, prior to recruitment, and identified through conserved transcriptional responses in mice and humans. Eur Heart J. 2015;36:1923–34. - PMC - PubMed
-
- Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619. - PubMed
-
- Nishida K, Taneike M, Otsu K. The role of autophagic degradation in the heart. J Mol Cell Cardiol. 2015;78:73–79. - PubMed
LinkOut - more resources
Full Text Sources
