Shikonin promotes osteogenesis and suppresses osteoclastogenesis in vitro
- PMID: 33437384
- PMCID: PMC7791499
Shikonin promotes osteogenesis and suppresses osteoclastogenesis in vitro
Abstract
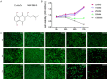
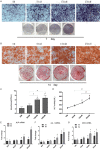
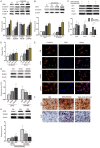
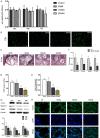
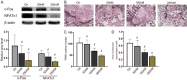
Shikonin, as a traditional Chinese herbal medicine with a role of anti-cancer, anti-inflammatory, anti-bacterial and other effects. However, there are few studies on the effect of shikonin on osteoporosis. Therefore, the purpose of this study aims to investigate the role and mechanism of shikonin on differentiation of BMSCs and BMMs into osteoblasts and osteoclasts formation. In our study, we treated the cells with different concentrations of shikonin, and then illuminated its effect on oteogenesis and osteoclast differentiation by ALP/alizarin red staining, ALP activity, qRT-PCR, immunofluorescence, Western blot, and TRAP staining. The result showed that shikonin may promote BMSCs differentiate into osteoblasts through the Wnt/β-catenin signaling pathway. At the same time, it may also inhibit the formation of osteoclasts mediated by RANK/RANKL/OPG pathway in vitro. Our research explains excellently the mechanism of shikonin alleviating osteoporosis in vitro, which maybe contributing to the exploration of a new way to prevent osteoporosis.
Keywords: OPG; RANKL; Shikonin; Wnt; mesenchymal stem cells; osteoporosis; β-cantenin.
AJTR Copyright © 2020.
Conflict of interest statement
None.
Figures
Similar articles
-
Oridonin promotes osteogenesis through Wnt/β-catenin pathway and inhibits RANKL-induced osteoclastogenesis in vitro.Life Sci. 2020 Dec 1;262:118563. doi: 10.1016/j.lfs.2020.118563. Epub 2020 Oct 7. Life Sci. 2020. PMID: 33038376
-
Shikonin relieves osteoporosis of ovariectomized mice by inhibiting RANKL-induced NF-κB and NFAT pathways.Exp Cell Res. 2020 Sep 1;394(1):112115. doi: 10.1016/j.yexcr.2020.112115. Epub 2020 May 28. Exp Cell Res. 2020. PMID: 32473224
-
Shikonin mitigates ovariectomy-induced bone loss and RANKL-induced osteoclastogenesis via TRAF6-mediated signaling pathways.Biomed Pharmacother. 2020 Jun;126:110067. doi: 10.1016/j.biopha.2020.110067. Epub 2020 Apr 6. Biomed Pharmacother. 2020. PMID: 32272431
-
[Review for treatment effect and signaling pathway regulation of kidney-tonifying traditional Chinese medicine on osteoporosis].Zhongguo Zhong Yao Za Zhi. 2018 Jan;43(1):21-30. doi: 10.19540/j.cnki.cjcmm.20171106.002. Zhongguo Zhong Yao Za Zhi. 2018. PMID: 29552807 Review. Chinese.
-
Osteoclast differentiation by RANKL and OPG signaling pathways.J Bone Miner Metab. 2021 Jan;39(1):19-26. doi: 10.1007/s00774-020-01162-6. Epub 2020 Oct 20. J Bone Miner Metab. 2021. PMID: 33079279 Review.
Cited by
-
Progress of Wnt Signaling Pathway in Osteoporosis.Biomolecules. 2023 Mar 6;13(3):483. doi: 10.3390/biom13030483. Biomolecules. 2023. PMID: 36979418 Free PMC article. Review.
-
Shikonin Attenuates Cochlear Spiral Ganglion Neuron Degeneration by Activating Nrf2-ARE Signaling Pathway.Front Mol Neurosci. 2022 Feb 24;15:829642. doi: 10.3389/fnmol.2022.829642. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35283722 Free PMC article.
-
A sustained-release Trametinib bio-multifunction hydrogel inhibits orthodontically induced inflammatory root resorption.RSC Adv. 2022 Jun 6;12(26):16444-16453. doi: 10.1039/d2ra00763k. eCollection 2022 Jun 1. RSC Adv. 2022. PMID: 35754868 Free PMC article.
-
Review of Shikonin and Derivatives: Isolation, Chemistry, Biosynthesis, Pharmacology and Toxicology.Front Pharmacol. 2022 Jul 1;13:905755. doi: 10.3389/fphar.2022.905755. eCollection 2022. Front Pharmacol. 2022. PMID: 35847041 Free PMC article. Review.
-
Hypoxia inducible factor-1α related mechanism and TCM intervention in process of early fracture healing.Chin Herb Med. 2023 Dec 14;16(1):56-69. doi: 10.1016/j.chmed.2023.09.006. eCollection 2024 Jan. Chin Herb Med. 2023. PMID: 38375046 Free PMC article. Review.
References
-
- Reid IR. Short-term and long-term effects of osteoporosis therapies. Nat Rev Endocrinol. 2015;11:418–428. - PubMed
-
- Barrett-Connor E, Grady D, Stefanick ML. The rise and fall of menopausal hormone therapy. Annu Rev Public Health. 2005;26:115–140. - PubMed
-
- Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang OH, Mitlak BH. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441. - PubMed
LinkOut - more resources
Full Text Sources
