Geographical pattern of genetic diversity in Capsella bursa-pastoris (Brassicaceae)-A global perspective
- PMID: 33437423
- PMCID: PMC7790636
- DOI: 10.1002/ece3.7010
Geographical pattern of genetic diversity in Capsella bursa-pastoris (Brassicaceae)-A global perspective
Abstract
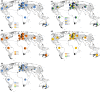
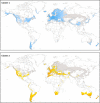
We analyzed the global genetic variation pattern of Capsella bursa-pastoris (Brassicaceae) as expressed in allozymic (within-locus) diversity and isozymic (between-locus) diversity. Results are based on a global sampling of more than 20,000 C. bursa-pastoris individuals randomly taken from 1,469 natural provenances in the native and introduced range, covering a broad spectrum of the species' geographic distribution. We evaluated data for population genetic parameters and F-statistics, and Mantel tests and AMOVA were performed. Geographical distribution patterns of alleles and multilocus genotypes are shown in maps and tables. Genetic diversity of introduced populations is only moderately reduced in comparison with native populations. Global population structure was analyzed with structure, and the obtained cluster affiliation was tested independently with classification approaches and macroclimatic data using species distribution modeling. Analyses revealed two main clusters: one distributed predominantly in warm arid to semiarid climate regions and the other predominantly in more temperate humid to semihumid climate regions. We observed admixture between the two lineages predominantly in regions with intermediate humidity in both the native and non-native ranges. The genetically derived clusters are strongly supported in macroclimatic data space. The worldwide distribution patterns of genetic variation in the range of C. bursa-pastoris can be explained by intensive intra- and intercontinental migration, but environmental filtering due to climate preadaption seems also involved. Multiple independent introductions of genotypes from different source regions are obvious. "Endemic" genotypes might be the outcome of admixture or of de novo mutation. We conclude that today's successfully established Capsella genotypes were preadapted and found matching niche conditions in the colonized range parts.
Keywords: Capsella bursa‐pastoris; adaptation; colonization; macroclimate; multilocus genotypes; species distribution model.
© 2020 The Authors. Ecology and Evolution published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures





References
-
- Brown, A. H. D. , Matheson, A. C. , & Eldridge, K. G. (1975). Estimation of the mating system of Eucalyptus obliqua L'Herit. by using allozyme polymorphisms. Australian Journal of Botany, 23(6), 931–949.
-
- Brown, A. H. D. , & Weir, B. S. (1983). Measuring genetic variability in plant populations In Tanksley S. D., & Ortin T. J. (Eds.), Isozymes in plant genetics and breeding, part A (pp. 219–239). Elsevier.
Associated data
LinkOut - more resources
Full Text Sources

