Sympatric divergence of the ergot fungus, Claviceps purpurea, populations infecting agricultural and nonagricultural grasses in North America
- PMID: 33437429
- PMCID: PMC7790621
- DOI: 10.1002/ece3.7028
Sympatric divergence of the ergot fungus, Claviceps purpurea, populations infecting agricultural and nonagricultural grasses in North America
Abstract
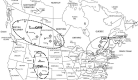
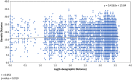
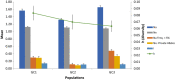
The ergot diseases of agricultural and nonagricultural grasses are caused by the infection of Claviceps spp. (Hypocreales, Ascomycota) on florets, producing dark spur-like sclerotia on spikes that are toxic to humans and animals, leading to detrimental impacts on agriculture and economy due to the downgrading of cereal grains, import-export barriers, reduced yield, and ecological concerns. At least seven phylogenetic lineages (phylogenetic species) were identified within the premolecular concept of C. purpurea s.l. (sensu lato) in agricultural areas and vicinities in Canada and the Western United States. Claviceps purpurea s.s (sensu stricto) remained as the most prevalent species with a wide host range, including cereal crops, native, invasive, and weedy grasses. The knowledge on genetic diversity and distribution of C. purpurea s.s. in North America is lacking. The objective of the present study was to shed light on genetic differentiation and evolution of the natural populations of C. purpurea s.s. Multilocus DNA sequences of samples from Canada and the Western USA were analyzed using a phylogenetic network approach, and population demographic parameters were investigated. Results showed that three distinct genetically subdivided populations exist, and the subdivision is not correlated with geographic or host differentiations. Potential intrinsic mechanisms that might play roles in leading to the cessation of gene flows among the subpopulations, that is, mating and/or vegetative incompatibility, genomic adaptation, were discussed. The neutrality of two house-keeping genes that are widely used for DNA barcoding, that is, translation elongation factor 1-α (TEF1-α) and RNA polymerase II second largest subunit (RPB2), was challenged and discussed.
Keywords: Ascomycota; house‐keeping gene; multilocus haplotype; neutrality; phylogenetic network; population structure; selective sweeping.
© 2020 Her Majesty the Queen in Right of Canada. Ecology and Evolution published by John Wiley & Sons Ltd.
Conflict of interest statement
No conflict.
Figures




References
-
- Alderman, S. C. , Halse, R. R. , & White, J. F. (2004). A reevaluation of the host range and geographical distribution of Claviceps species in the United States. Plant Disease, 88, 63–81. - PubMed
-
- Bouckaert, R. R. , & Heled, J. (2014). DensiTree 2: Seeing trees through the forest. bioRxiv, 012401 10.1101/012401 - DOI
-
- Bouckaert, R. , Vaughan, T. G. , Barido‐Sottani, J. , Duchêne, S. , Fourment, M. , Gavryushkina, A. , Heled, J. , Jones, G. , Kühnert, D. , De Maio, N. , Matschiner, M. , Mendes, F. K. , Müller, N. F. , Ogilvie, H. A. , du Plessis, L. , Popinga, A. , Rambaut, A. , Rasmussen, D. , Siveroni, I. , … Drummond, A. J. (2019). BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLOS Computational Biology, 15(4), e1006650 10.1371/journal.pcbi.1006650 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

