The AndroCoV Clinical Scoring for COVID-19 Diagnosis: A Prompt, Feasible, Costless, and Highly Sensitive Diagnostic Tool for COVID-19 Based on a 1757-Patient Cohort
- PMID: 33437562
- PMCID: PMC7793341
- DOI: 10.7759/cureus.12565
The AndroCoV Clinical Scoring for COVID-19 Diagnosis: A Prompt, Feasible, Costless, and Highly Sensitive Diagnostic Tool for COVID-19 Based on a 1757-Patient Cohort
Abstract
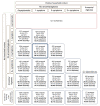
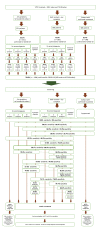
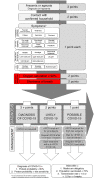
Introduction A major barrier for successful therapeutic approaches for COVID-19 is the inability to diagnose COVID-19 during the viral replication stage, when drugs with potential antiviral activity could demonstrate efficacy and preclude progression to more severe stages. Reasons that hamper an earlier diagnosis of COVID-19 include the unspecific and mild symptoms during the first stage, the delay in the diagnosis and specific management caused by the requirement of a real-time reverse transcriptase-polymerase chain reaction (RT-PCR) for SARS-CoV-2 for the diagnosis of COVID-19, and the insufficient sensitivity of the RT-PCR-SARS-CoV-2, converse to what is recommended for a screening test during an outbreak. More sensitive and earlier diagnostic tools for COVID-19 should be unraveled as a key strategy for a breakthrough change in the disease course and response to specific therapies, particularly those that target the blockage of viral shedding. We aimed to create an accurate, sensitive, easy-to-perform, and intuitive clinical scoring for the diagnosis of COVID-19 without the need for an RT-PCR-SARS-CoV-2 (termed The AndroCoV Clinical Scoring for COVID-19 Diagnosis), resulting from a 1,757 population cohort, to eventually encourage the management of patients with a high pre-clinical likelihood of presenting COVID-19, independent of an RT-PCR-SARS-COV-2 test, to avoid delays and loss of appropriate timing for potential therapies. Methods This is a post-hoc analysis of clinical data prospectively collected of the Pre-AndroCoV and AndroCov Trials, which resulted in scorings for the clinical diagnosis of COVID-19 based on the likelihood of presenting with actual COVID-19 according to the number of symptoms, presence of anosmia, and known positive household contact. Sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio, and accuracy were calculated for subjects screened in two different periods and both periods together, for females, males, and both, in a total of nine different scenarios, according to combinations of one, two, or three or more symptoms or the presence of anosmia in subjects without known positive household contacts, and no symptoms, one, two, or three or more symptoms, or presence of anosmia or ageusia in subjects with known positive household contacts. Scorings that yielded the highest pre-test probability, sensitivity, and accuracy were selected. Results Of the 1,757 patients screened, 1,284 were diagnosed with COVID-19. The scoring that required: (1) two or more symptoms, or anosmia or ageusia alone, for subjects without known contact; or (2) one or more symptoms, including anosmia or ageusia alone, when with known positive contacts presented the highest accuracy (80.4%) among all combinations attempted, and higher sensitivity (85.7%) than RT-PCR-SARS-CoV-2 commercially available kit tests. Conclusion The AndroCoV clinical scoring for COVID-19 diagnosis was demonstrated to be a feasible, easy, costless, and sensitive diagnostic tool for the clinical diagnosis of COVID-19. Because the clinical diagnosis of COVID-19 avoids delays in specific treatments, particularly for high-risk populations, prevents false-negative diagnosis, and reduces diagnostic costs, this diagnostic tool should be considered as an option for COVID-19 diagnosis, at least while SARS-CoV-2 is the prevailing circulating virus and vaccination rate is below the required for herd immunity.
Keywords: ageusia; anosmia; clinical diagnosis of covid-19; covid-19; covid-19 diagnosis; diagnosis; pandemic; rtpcr; rtpcr-sars-cov-2; sars-cov-2.
Copyright © 2021, Cadegiani et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- The trinity of COVID-19: immunity, inflammation and intervention. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. http://10.1038/s41577-020-0311-8. Nat Rev Immunol. 2020;20:363–374. - PMC - PubMed
-
- Azithromycin with nitazoxanide, hydroxychloroquine or ivermectin, with or without dutasteride, for early stage COVID- 19: an open-label prospective observational study in males with mild-to-moderate COVID-19 (The Pre-AndroCoV Male Trial) [PREPRINT] Cadegiani FA, Goren A, Wambier CG, McCoy J. Research Square. 2020
-
- Early COVID-19 therapy with azithromycin plus nitazoxanide, ivermectin or hydroxychloroquine in outpatient settings significantly reduced symptoms compared to known outcomes in untreated patients [PREPRINT] Cadegiani FA, Goren A, Wambier CG, McCoy J. https://doi.org/10.1101/2020.10.31.20223883 medRxiv. 2020 - PMC - PubMed
-
- An open-label prospective observational study of antiandrogen and non-antiandrogen early pharmacological approaches in females with mild-to-moderate COVID-19. The Pre-AndroCoV female trial [PREPRINT] Cadegiani FA, Goren A, Wambier CG, McCoy J. https://doi.org/10.1101/2020.10.05.20206870 medRxiv. 2020
-
- Hydroxychloroquine, nitazoxanide and ivermectin have similar effects in early COVID- 19: a head-to-head comparison of the Pre-AndroCoV trial. Cadegiani FA, Goren A, McCoy J, et al. https://www.researchsquare.com/article/rs-98106/v1 Research Square. 2020
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
