High Frequency Sonoprocessing: A New Field of Cavitation-Free Acoustic Materials Synthesis, Processing, and Manipulation
- PMID: 33437572
- PMCID: PMC7788597
- DOI: 10.1002/advs.202001983
High Frequency Sonoprocessing: A New Field of Cavitation-Free Acoustic Materials Synthesis, Processing, and Manipulation
Abstract
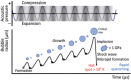
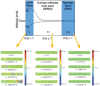
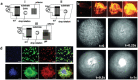
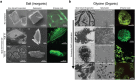
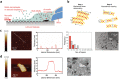
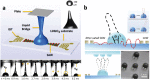
Ultrasound constitutes a powerful means for materials processing. Similarly, a new field has emerged demonstrating the possibility for harnessing sound energy sources at considerably higher frequencies (10 MHz to 1 GHz) compared to conventional ultrasound (⩽3 MHz) for synthesizing and manipulating a variety of bulk, nanoscale, and biological materials. At these frequencies and the typical acoustic intensities employed, cavitation-which underpins most sonochemical or, more broadly, ultrasound-mediated processes-is largely absent, suggesting that altogether fundamentally different mechanisms are at play. Examples include the crystallization of novel morphologies or highly oriented structures; exfoliation of 2D quantum dots and nanosheets; polymer nanoparticle synthesis and encapsulation; and the possibility for manipulating the bandgap of 2D semiconducting materials or the lipid structure that makes up the cell membrane, the latter resulting in the ability to enhance intracellular molecular uptake. These fascinating examples reveal how the highly nonlinear electromechanical coupling associated with such high-frequency surface vibration gives rise to a variety of static and dynamic charge generation and transfer effects, in addition to molecular ordering, polarization, and assembly-remarkably, given the vast dimensional separation between the acoustic wavelength and characteristic molecular length scales, or between the MHz-order excitation frequencies and typical THz-order molecular vibration frequencies.
Keywords: crystallization; molecular self‐assembly; nanomaterials; polymers; sonochemistry; surface acoustic waves.
© 2020 The Authors. Published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Suslick K. S., Science 1990, 247, 1439. - PubMed
-
- Thompson L. H., Doraiswamy L. K., Ind. Eng. Chem. Res. 1999, 38, 1215.
-
- Margulis M. A., Margulis I. M., Ultrason. Sonochem. 2002, 9, 1. - PubMed
-
- Suslick K. S., Flannigan D. J., Annu. Rev. Phys. Chem. 2008, 59, 659. - PubMed
-
- Wood R. J., Lee J., Bussemaker M. J., Ultrason. Sonochem. 2017, 38, 351. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
