One strike loading organ culture model to investigate the post-traumatic disc degenerative condition
- PMID: 33437633
- PMCID: PMC7773974
- DOI: 10.1016/j.jot.2020.08.003
One strike loading organ culture model to investigate the post-traumatic disc degenerative condition
Abstract
Background: Acute trauma on intervertebral discs (IVDs) is thought to be one of the risk factors for IVD degeneration. The pathophysiology of IVD degeneration induced by single high impact mechanical injury is not very well understood. The aim of this study was using a post-traumatic IVD model in a whole organ culture system to analyze the biological and biomechanical consequences of the single high-impact loading event on the cultured IVDs.
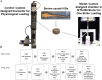
Methods: Isolated healthy bovine IVDs were loaded with a physiological loading protocol in the control group or with injurious loading (compression at 50% of IVD height) in the one strike loading (OSL) group. After another 1 day (short term) or 8 days (long term) of whole organ culture within a bioreactor, the samples were collected to analyze the cell viability, histological morphology and gene expression. The conditioned medium was collected daily to analyze the release of glycosaminoglycan (GAG) and nitric oxide (NO).
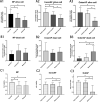
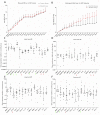
Results: The OSL IVD injury group showed signs of early degeneration including reduction of dynamic compressive stiffness, annulus fibrosus (AF) fissures and extracellular matrix degradation. Compared to the control group, the OSL model group showed more severe cell death (P < 0.01) and higher GAG release in the culture medium (P < 0.05). The MMP and ADAMTS families were up-regulated in both nucleus pulposus (NP) and AF tissues from the OSL model group (P < 0.05). The OSL injury model induced a traumatic degenerative cascade in the whole organ cultured IVD.
Conclusions: The present study shows a single hyperphysiological mechanical compression applied to healthy bovine IVDs caused significant drop of cell viability, altered the mRNA expression in the IVD, and increased ECM degradation. The OSL IVD model could provide new insights into the mechanism of mechanical injury induced early IVD degeneration.
The translational potential of this article: This model has a high potential for investigation of the degeneration mechanism in post-traumatic IVD disease, identification of novel biomarkers and therapeutic targets, as well as screening of treatment therapies.
Keywords: Degeneration; Extracellular matrix; Intervertebral disc; Mechanical injury; One strike loading; Post-traumatic.
© 2020 The Authors.
Conflict of interest statement
The authors have no conflicts of interest to disclose in relation to this article.
Figures



Similar articles
-
Repetitive strikes loading organ culture model to investigate the biological and biomechanical responses of the intervertebral disc.JOR Spine. 2024 Jan 19;7(1):e1314. doi: 10.1002/jsp2.1314. eCollection 2024 Mar. JOR Spine. 2024. PMID: 38249719 Free PMC article.
-
Programmed NP Cell Death Induced by Mitochondrial ROS in a One-Strike Loading Disc Degeneration Organ Culture Model.Oxid Med Cell Longev. 2021 Aug 31;2021:5608133. doi: 10.1155/2021/5608133. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34512867 Free PMC article.
-
Functional impact of integrin α5β1 on the homeostasis of intervertebral discs: a study of mechanotransduction pathways using a novel dynamic loading organ culture system.Spine J. 2015 Mar 1;15(3):417-26. doi: 10.1016/j.spinee.2014.12.143. Epub 2014 Dec 27. Spine J. 2015. PMID: 25546513 Free PMC article.
-
Intervertebral disc organ culture for the investigation of disc pathology and regeneration - benefits, limitations, and future directions of bioreactors.Connect Tissue Res. 2020 May-Jul;61(3-4):304-321. doi: 10.1080/03008207.2019.1665652. Epub 2019 Sep 26. Connect Tissue Res. 2020. PMID: 31556329 Review.
-
Mechanobiology of annulus fibrosus and nucleus pulposus cells in intervertebral discs.Cell Tissue Res. 2020 Mar;379(3):429-444. doi: 10.1007/s00441-019-03136-1. Epub 2019 Dec 17. Cell Tissue Res. 2020. PMID: 31844969 Review.
Cited by
-
Kartogenin-enhanced dynamic hydrogel ameliorates intervertebral disc degeneration via restoration of local redox homeostasis.J Orthop Translat. 2023 Jul 31;42:15-30. doi: 10.1016/j.jot.2023.07.002. eCollection 2023 Sep. J Orthop Translat. 2023. PMID: 37560412 Free PMC article.
-
Multiaxial rotational loading compromises the transition zone of the intervertebral disc: Ex vivo study using next-generation bioreactors.Bioeng Transl Med. 2025 Jun 8;10(4):e70033. doi: 10.1002/btm2.70033. eCollection 2025 Jul. Bioeng Transl Med. 2025. PMID: 40708985 Free PMC article.
-
Investigating the physiological relevance of ex vivo disc organ culture nutrient microenvironments using in silico modeling and experimental validation.JOR Spine. 2021 Mar 2;4(2):e1141. doi: 10.1002/jsp2.1141. eCollection 2021 Jun. JOR Spine. 2021. PMID: 34337330 Free PMC article.
-
Noninvasive multimodal fluorescence and magnetic resonance imaging of whole-organ intervertebral discs.Biomed Opt Express. 2021 May 7;12(6):3214-3227. doi: 10.1364/BOE.421205. eCollection 2021 Jun 1. Biomed Opt Express. 2021. PMID: 34221655 Free PMC article.
-
In Situ Cell Signalling of the Hippo-YAP/TAZ Pathway in Reaction to Complex Dynamic Loading in an Intervertebral Disc Organ Culture.Int J Mol Sci. 2021 Dec 20;22(24):13641. doi: 10.3390/ijms222413641. Int J Mol Sci. 2021. PMID: 34948441 Free PMC article.
References
-
- Ricci J.A., Stewart W.F., Chee E., Leotta C., Foley K., Hochberg M.C. Back pain exacerbations and lost productive time costs in United States workers. Spine. 2006;31(26):3052–3060. - PubMed
-
- Wang H., Dwyer-Lindgren L., Lofgren K.T., Rajaratnam J.K., Marcus J.R., Levin-Rector A. Age-specific and sex-specific mortality in 187 countries, 1970-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2071–2094. - PubMed
-
- Vergroesen P.P., Kingma I., Emanuel K.S., Hoogendoorn R.J., Welting T.J., van Royen B.J. Mechanics and biology in intervertebral disc degeneration: a vicious circle. Osteoarthritis Cartilage. 2015;23(7):1057–1070. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

