A novel tissue-engineered bone graft composed of silicon-substituted calcium phosphate, autogenous fine particulate bone powder and BMSCs promotes posterolateral spinal fusion in rabbits
- PMID: 33437634
- PMCID: PMC7773983
- DOI: 10.1016/j.jot.2020.06.003
A novel tissue-engineered bone graft composed of silicon-substituted calcium phosphate, autogenous fine particulate bone powder and BMSCs promotes posterolateral spinal fusion in rabbits
Abstract
Background: Autogenous bone graft is the gold standard bone grafting substrate available in spinal fusion because of its osteoconductive, osteogenic, and osteoinductive properties. However, several shortcomings including bleeding, infection, chronic pain, and nerve injury are known to be associated with the procedure. Bone tissue engineering has emerged as an alternative therapeutic strategy for bone grafts. New materials have been developed and tested that can substitute for the autogenous bone grafts used in the spinal fusion. The purpose of this study is to evaluate the role of a novel tissue-engineered bone graft with silicon-substituted calcium phosphate (Si-CaP), autogenous fine particulate bone powder (AFPBP), and bone marrow mesenchymal stem cells (BMSCs) using a rabbit posterolateral lumbar fusion model based on bone tissue engineering principles. The application of this graft can represent a novel choice for autogenous bone to reduce the amount of autogenous bone and promote spinal fusion.
Methods: BMSCs from New Zealand white rabbits were isolated and cultured in vitro. Then, BMSCs were marked by the cell tracker chloromethyl-benzamidodialkylcarbocyanine (CM-Dil). A total of 96 New Zealand White rabbits were randomly divided into four groups: (a) AFPBP, (b) Si-CaP, (c) Si-CaP/AFPBP, (d) Si-CaP/AFPBP/BMSCs.The rabbits underwent bilateral posterolateral spine arthrodesis of the L5-L6 intertransverse processes using different grafts. Spinal fusion and bone formation were evaluated at 4, 8, and 12 weeks after surgery by manual palpation, radiology, micro-computed tomography (micro-CT), histology, and scanning electronic microscopy (SEM).
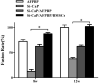
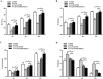
Results: The rate of fusion by manual palpation was higher in the Si-CaP/AFPBP/BMSCs group than the other groups at 8 weeks. The fusion rates in the Si-CaP/AFPBP/BMSCs and the AFPBP groups both reached 100%, which was higher than the Si-CaP/AFPBP group (62.5%) (P > 0.05) and Si-CaP group (37.5%) (P < 0.05) at 12 weeks. New bone formation was observed in all groups after implantation by radiology and micro-CT. The radiographic and CT scores increased in all groups from 4 to 12 weeks, indicating a time-dependent osteogenetic process. The Si-CaP/AFPBP/BMSCs group showed a larger amount of newly formed bone than the Si-CaP/AFPBP and Si-CaP groups at 12 weeks. Bone formation in the Si-CaP/AFPBP/BMSCs group was similar to the AFPBP group. Histology showed that new bone formation continued and increased along with the degradation and absorption of Si-CaP and AFPBP from 4 to 12 weeks in the Si-CaP, Si-CaP/AFPBP, and Si-CaP/AFPBP/BMSCs groups. At 4 weeks, a higher proportion of bone was detected in the AFPBP group (23.49%) compared with the Si-CaP/AFPBP/BMSCs group (14.66%, P < 0.05). In the Si-CaP/AFPBP/BMSCs group at 8 weeks, the area percentage of new bone formation was 28.56%, which was less than the AFPBP group (33.21%, P < 0.05). No difference in bone volume was observed between the Si-CaP/AFPBP/BMSCs group (44.39%) and AFPBP group (45.06%) at 12 weeks (P > 0.05). At 12 weeks, new trabecular were visible in the Si-CaP/AFPBP/BMSCs group by SEM. CM-Dil-positive cells were observed at all stages. Compared with histological images, BMSCs participate in various stages of osteogenesis by transforming into osteoblasts, chondrocytes, and osteocytes.
Conclusion: This study demonstrated for the first time that Si-CaP/AFPBP/BMSCs is a novel tissue-engineered bone graft with excellent bioactivity, biocompatibility, and biodegradability. The graft could reduce the amount of autogenous bone and promote spinal fusion in a rabbit posterolateral lumbar fusion model, representing a novel alternative to autogenous bone.
The translational potential of this article: The translational potential of this article lies in that this graft will be a novel spinal fusion graft with great potential for clinical applications.
Keywords: Autogenous fine particulate bone powder; Bone marrow mesenchymal stem cells; Bone tissue engineering; Silicate-substituted calcium phosphate; Spinal fusion.
© 2020 The Author(s).
Conflict of interest statement
The authors have no conflicts of interest to disclose in relation to this article.
Figures





Similar articles
-
The efficacy of a nanosynthetic bone graft substitute as a bone graft extender in rabbit posterolateral fusion.Spine J. 2021 Nov;21(11):1925-1937. doi: 10.1016/j.spinee.2021.05.017. Epub 2021 May 23. Spine J. 2021. PMID: 34033931
-
Engineered periosteum-bone biomimetic bone graft enhances posterolateral spine fusion in a rabbit model.Spine J. 2019 Apr;19(4):762-771. doi: 10.1016/j.spinee.2018.09.013. Epub 2018 Sep 25. Spine J. 2019. PMID: 30266454
-
Comparison of Healos/bone marrow to INFUSE(rhBMP-2/ACS) with a collagen-ceramic sponge bulking agent as graft substitutes for lumbar spine fusion.Spine (Phila Pa 1976). 2005 May 1;30(9):1001-7; discussion 1007. doi: 10.1097/01.brs.0000160997.91502.3b. Spine (Phila Pa 1976). 2005. PMID: 15864149
-
Experimental posterolateral lumbar spinal fusion with a demineralized bone matrix gel.Spine (Phila Pa 1976). 1998 Jan 15;23(2):159-67. doi: 10.1097/00007632-199801150-00003. Spine (Phila Pa 1976). 1998. PMID: 9474720 Review.
-
Recent Research Advances in Biologic Bone Graft Materials for Spine Surgery.Curr Rev Musculoskelet Med. 2020 Jun;13(3):318-325. doi: 10.1007/s12178-020-09620-4. Curr Rev Musculoskelet Med. 2020. PMID: 32323248 Free PMC article. Review.
Cited by
-
An innovative self-stabilised 3D-printed artificial vertebral body designed for clinical application and comparison with the conventional implants.J Orthop Translat. 2025 Jun 5;53:52-62. doi: 10.1016/j.jot.2025.04.010. eCollection 2025 Jul. J Orthop Translat. 2025. PMID: 40529899 Free PMC article.
-
Medicine and models of degenerative orthopaedic disorders.J Orthop Translat. 2020 Dec 23;26:1-2. doi: 10.1016/j.jot.2020.12.002. eCollection 2021 Jan. J Orthop Translat. 2020. PMID: 33437617 Free PMC article. No abstract available.
-
Biodegradable Cements for Bone Regeneration.J Funct Biomater. 2023 Feb 27;14(3):134. doi: 10.3390/jfb14030134. J Funct Biomater. 2023. PMID: 36976058 Free PMC article. Review.
-
Periosteum and development of the tissue-engineered periosteum for guided bone regeneration.J Orthop Translat. 2022 Feb 16;33:41-54. doi: 10.1016/j.jot.2022.01.002. eCollection 2022 Mar. J Orthop Translat. 2022. PMID: 35228996 Free PMC article. Review.
-
In Vitro Evaluation of Biphasic Calcium Phosphate Scaffolds Derived from Cuttlefish Bone Coated with Poly(ester urea) for Bone Tissue Regeneration.Polymers (Basel). 2023 May 10;15(10):2256. doi: 10.3390/polym15102256. Polymers (Basel). 2023. PMID: 37242831 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

