Rhus semialata M. extract ameliorate para-phenylenediamine-induced toxicity in keratinocytes
- PMID: 33437652
- PMCID: PMC7786012
- DOI: 10.1016/j.toxrep.2020.12.020
Rhus semialata M. extract ameliorate para-phenylenediamine-induced toxicity in keratinocytes
Abstract
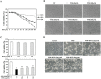
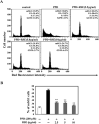
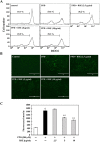
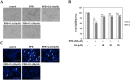
para-Phenylediamine (PPD), a major component of hair dyeing ingredients, can induce allergenic sensitization and exert mutagenic, tumorigenic and cytotoxic effect. In this study, we determined the cytotoxic effect of PPD on human keratinocytes and evaluated the protective effect of Rhus semialata M. extracts (RSE) on PPD induced cytotoxicity for the first time. We observed that RSE is a strong inhibitory agent against PPD-induced toxicity in human keratinocytes. The results indicated that RSE pretreatment significantly could suppress PPD induced cytotoxic effects, including decrease of cell viability, accumulation in subG1 phase of cells, and relocation of phosphatidylserine on keratinocytes. Also, we found that PPD caused cytotoxicity was associated with mitochondrial membrane potential loss and subsequent activation of caspase and PARP degradation. However, pretreatment of RSE showed preventive activities against PPD induced mitochondrial membrane potential loss and ROS production in keratinocytes. In conclusion, the results of present study suggest that RSE was able to protect the skin from several cytotoxic effects of PPD and could be a meaningful material in many industries using PPD.
Keywords: Apoptosis; DCFH-DA, 2',7'-dichlorodihydrofluorescein diacetate; DMEM, Dulbecco’s modified Eagle’s medium; DMSO, Dimethyl sulfoxide; DiOC6, 3,3'dihexyloxacarbocyanine iodide; FBS, Fetal bovine serum; Keratinocytes; MTT, 3-[4,5-Dimethyl-2-thiazolyl]-2,5-diphenyl-2H-tetrazolium bromide; Mitochondrial damage; PI, Propidium iodide; PPD, para-Phenylenediamine; ROS, Reactive oxygen species; RSE, Rhus semialata M extracts; Rhus semialata M; para-Phenylenediamine.
© 2020 Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Dressler W.E., Appelqvist T. Plasma/blood pharmacokinetics and metabolism after dermal exposure to para-aminophenol or para-phenylenediamine. Food Chem. Toxicol. 2006;44:371–379. - PubMed
-
- Diepgen T.L., Naldi L., Bruze M., Cazzaniga S., Schuttelaar M.L., Elsner P., Goncalo M., Ofenloch R., Svensson A. Prevalance of contact allergy to p-phenylenediamine in the European general population. J. Invest. Dermatol. 2016;136:409–415. - PubMed
-
- Hamann D., Yazar K., Hamann C.R., Thyssen J.P., Lidén C. p-Phenylenediamine and other allergens in hair dye products in the United States: a consumer exposure study. Contact Derm. 2014;70:213–218. - PubMed
-
- Thyssen J.P., White J.M. Epidemiological data on consumer allergy to p-phenylenediamine. Contact Derm. 2008;59:327–343. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

