A study protocol for expanding the screening interval of endoscopic screening for gastric cancer based on individual risks: prospective cohort study of gastric cancer screening
- PMID: 33437803
- PMCID: PMC7791261
- DOI: 10.21037/atm-20-5949
A study protocol for expanding the screening interval of endoscopic screening for gastric cancer based on individual risks: prospective cohort study of gastric cancer screening
Abstract
Background: The Japanese government has recommended a 2-year endoscopic screening interval for gastric cancer. However, insufficient resources have constrained participation in endoscopic screening for gastric cancer. One way to avoid endoscopic screening harms and provide equal access is to define the appropriate screening interval.
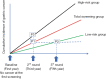
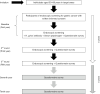
Methods: To expand screening interval from more than 2 years for low-risk group, a single-arm cohort of endoscopic screening started. At the baseline screening, the participants underwent endoscopic screening for gastric cancer, Helicobacter pylori (H. pylori) antibody test, and serum pepsinogen test (first year), and followed after 2 and 4 years (within the first 5 years). We also assessed H. pylori infection and atrophy status on images of upper gastrointestinal endoscopy at the baseline. A new screening model will be developed by dividing the participants into high-risk and low-risk groups based on demographics, history of H. pylori eradication, serological testing, and endoscopic diagnosis. The cumulative gastric cancer incidence after negative results at baseline are compared between the low-risk group on the 3rd screening round after 4 years from baseline and the total screening group on the 2nd screening round after 2 years. If the cumulative gastric cancer incidence in the low-risk group on the 3rd screening round is lower than that in the total screening group on the 2nd screening round, the screening interval can be expanded to 4 years in the low-risk group.
Discussion: To reduce mortality from gastric cancer, a high participation rate of the target population is required. The screening interval of endoscopic screening can be changed if the individual risks for H. pylori infection are clarified. Our goal in this study is to obtain relevant data that can be used to improve the efficient use of endoscopic screening for gastric cancer by referring to individual risks in Japan.
Trial registration: UMIN000025839 (University Hospital Medical Information Network, Japan).
Keywords: Gastric cancer; Helicobacter pylori; atrophic gastritis; cancer screening; endoscopic screening; screening interval.
2020 Annals of Translational Medicine. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-5949). The authors have no conflicts of interest to declare.
Figures
References
-
- National Cancer Center [Internet]. Center for Cancer Control and Information Services. 2019. Available online: https://ganjoho.jp/public/index.html.
-
- Ministry of Health, Labour and Welfare [Internet]. Japan: Report of Health Promotion and Community Health Survey 2017. [cited 2020 Jun 30]. Available online: https://www.e-stat.go.jp/stat-search/files?page=1&toukei=00450025&tstat=....
-
- Ministry of Health, Labour and Welfare [Internet]. Report of Medical Institution Survey 2016. [cited 2020 Jun 30]. Available from: https://www.e-stat.go.jp/stat-search/files?page=1&toukei=00450021&tstat=....
LinkOut - more resources
Full Text Sources
