A pilot randomized controlled trial (RCT) of daily versus weekly interactive voice response calls to support adherence among antiretroviral treatment patients in India
- PMID: 33437832
- PMCID: PMC7793015
- DOI: 10.21037/mhealth-19-248a
A pilot randomized controlled trial (RCT) of daily versus weekly interactive voice response calls to support adherence among antiretroviral treatment patients in India
Abstract
Background: There are more than two million people living with HIV (PLH) in India, with more than 30% on antiretroviral treatment (ART) estimated to be non-adherent. This study aimed to (I) document adherence rates and related factors among ART patients in a large ART clinic in India, and (II) pilot test daily and weekly interactive voice response (IVR) calls to improve ART adherence and related outcomes.
Methods: ART patients reporting missing at least one dose in prior 6 months (N=362) were enrolled and assessed via self-report and medical record review. Participants were randomized to one of two conditions: (I) twice-daily IVR call reminders with self-management support messaging, plus a weekly IVR adherence assessment; or (II) an attention control, with only weekly IVR adherence assessment. Participants completed study assessments at baseline, 2-, 4-, and 6-months with high retention (88% to 96%).
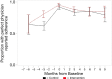
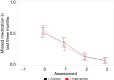
Results: Intention-to-treat analyses found limited support for intervention effects for improving or maintaining ART adherence or CD4 counts between the two study arms over 6-months follow-up. Adherence increased significantly in the six months prior to baseline from about 65% to >95% with perfect adherence based on pill counts from medical records and consistent with patient self-report measures, which presented ceiling effects for detecting improvements in ART adherence in response to IVR intervention exposure. There was also limited support for intervention effects on secondary, self-management outcomes.
Conclusions: High levels of adherence were sustained throughout the 6-month RCT. IVR regulation changes in India delayed study launch for 6 months, which likely allowed mobilization of improved adherence at the clinic, provider and patient levels in anticipation of the study launch. Therefore, ceiling effects limited inferences on intervention effects to improve adherence. Results suggest that clinic-level adherence monitoring may be sufficient to mobilize adherence improvements by providers and patients.
Trial registration: ClinicalTrials.gov registration #NCT02118454.
Keywords: HIV; India; antiretroviral therapy adherence; interactive voice response; mhealth.
2020 mHealth. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/mhealth-19-248a). The series “Digital Interventions for Hard-to-reach Populations” was commissioned by the editorial office without any funding or sponsorship. DS reports grants from National Institute of Allergy and Infectious Diseases, during the conduct of the study. AF reports grants from UCLA Semel Institute for Neuroscience and Human Behavior (T32MH109205), grants from University of California Global Health Institute (D43TW009343), during the conduct of the study. The authors have no other conflicts of interest to declare.
Figures
Similar articles
-
An Interactive Voice Response Software to Improve the Quality of Life of People Living With HIV in Uganda: Randomized Controlled Trial.JMIR Mhealth Uhealth. 2021 Feb 11;9(2):e22229. doi: 10.2196/22229. JMIR Mhealth Uhealth. 2021. PMID: 33570497 Free PMC article. Clinical Trial.
-
Development and Pilot Testing of Daily Interactive Voice Response (IVR) Calls to Support Antiretroviral Adherence in India: A Mixed-Methods Pilot Study.AIDS Behav. 2015 Jun;19 Suppl 2(0 2):142-55. doi: 10.1007/s10461-014-0983-9. AIDS Behav. 2015. PMID: 25638037 Free PMC article.
-
A Mobile Health Intervention Supporting Heart Failure Patients and Their Informal Caregivers: A Randomized Comparative Effectiveness Trial.J Med Internet Res. 2015 Jun 10;17(6):e142. doi: 10.2196/jmir.4550. J Med Internet Res. 2015. PMID: 26063161 Free PMC article. Clinical Trial.
-
Effectiveness of short message services and voice call interventions for antiretroviral therapy adherence and other outcomes: A systematic review and meta-analysis.PLoS One. 2018 Sep 21;13(9):e0204091. doi: 10.1371/journal.pone.0204091. eCollection 2018. PLoS One. 2018. PMID: 30240417 Free PMC article.
-
A Systematic Review of Mobile Phone Interventions (SMS/IVR/Calls) to Improve Adherence and Retention to Antiretroviral Treatment in Low-and Middle-Income Countries.AIDS Patient Care STDS. 2020 Feb;34(2):59-71. doi: 10.1089/apc.2019.0181. AIDS Patient Care STDS. 2020. PMID: 32049555
Cited by
-
How the Term 'Self-Management' is Used in HIV Research in Low- and Middle-Income Countries: A Scoping Review.AIDS Behav. 2022 Oct;26(10):3386-3399. doi: 10.1007/s10461-022-03668-8. Epub 2022 Apr 16. AIDS Behav. 2022. PMID: 35429310
-
Exploring the potential of telemedicine for improved primary healthcare in India: a comprehensive review.Lancet Reg Health Southeast Asia. 2024 Jun 8;27:100431. doi: 10.1016/j.lansea.2024.100431. eCollection 2024 Aug. Lancet Reg Health Southeast Asia. 2024. PMID: 38957222 Free PMC article. Review.
-
Effectiveness and acceptability of conversational agents for sexual health promotion: a systematic review and meta-analysis.Sex Health. 2022 Oct;19(5):391-405. doi: 10.1071/SH22016. Sex Health. 2022. PMID: 35863761 Free PMC article.
-
A Situational Analysis of the Impact of the COVID-19 Pandemic on Digital Health Research Initiatives in South Asia.Cureus. 2023 Nov 17;15(11):e48977. doi: 10.7759/cureus.48977. eCollection 2023 Nov. Cureus. 2023. PMID: 38111408 Free PMC article. Review.
References
-
- Joint United Nations Programme on HIV/AIDS (UNAIDS). Ending AIDS: Progress Towards the 90-90-90 Targets. 2017.
-
- Joint United Nations Programme on HIV/AIDS (UNAIDS). UNAIDS Data 2017. 2018. - PubMed
-
- Ministry of Health and Family Welfare, Department of AIDS Control. Antiretroviral therapy guidelines for HIV-infected adults and adolescents including post-exposure prophylaxis. 2007.
-
- National AIDS Control Organization (NACO). Annual Report 2016-17. 2017.
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous