Improved accuracy of cerebral blood flow quantification in the presence of systemic physiology cross-talk using multi-layer Monte Carlo modeling
- PMID: 33437846
- PMCID: PMC7779997
- DOI: 10.1117/1.NPh.8.1.015001
Improved accuracy of cerebral blood flow quantification in the presence of systemic physiology cross-talk using multi-layer Monte Carlo modeling
Erratum in
-
Erratum: Improved accuracy of cerebral blood flow quantification in the presence of systemic physiology cross-talk using multi-layer Monte Carlo modeling.Neurophotonics. 2022 Jan;9(1):019801. doi: 10.1117/1.NPh.9.1.019801. Epub 2022 Feb 17. Neurophotonics. 2022. PMID: 35233436 Free PMC article.
Abstract
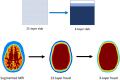
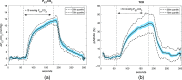
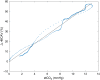
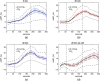
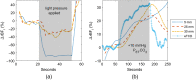
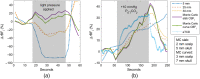
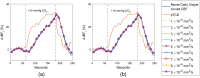
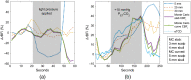
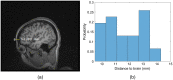
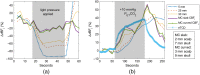
Significance: Contamination of diffuse correlation spectroscopy (DCS) measurements of cerebral blood flow (CBF) due to systemic physiology remains a significant challenge in the clinical translation of DCS for neuromonitoring. Tunable, multi-layer Monte Carlo-based (MC) light transport models have the potential to remove extracerebral flow cross-talk in cerebral blood flow index ( ) estimates. Aim: We explore the effectiveness of MC DCS models in recovering accurate changes in the presence of strong systemic physiology variations during a hypercapnia maneuver. Approach: Multi-layer slab and head-like realistic (curved) geometries were used to run MC simulations of photon propagation through the head. The simulation data were post-processed into models with variable extracerebral thicknesses and used to fit DCS multi-distance intensity autocorrelation measurements to estimate timecourses. The results of the MC values from a set of human subject hypercapnia sessions were compared with values estimated using a semi-infinite analytical model, as commonly used in the field. Results: Group averages indicate a gradual systemic increase in blood flow following a different temporal profile versus the expected rapid CBF response. Optimized MC models, guided by several intrinsic criteria and a pressure modulation maneuver, were able to more effectively separate changes from scalp blood flow influence than the analytical fitting, which assumed a homogeneous medium. Three-layer models performed better than two-layer ones; slab and curved models achieved largely similar results, though curved geometries were closer to physiological layer thicknesses. Conclusion: Three-layer, adjustable MC models can be useful in separating distinct changes in scalp and brain blood flow. Pressure modulation, along with reasonable estimates of physiological parameters, can help direct the choice of appropriate layer thicknesses in MC models.
Keywords: Monte Carlo; cerebral blood flow; diffuse correlation spectroscopy; hypercapnia; multi-layer.
© 2021 The Authors.
Figures


References
-
- Tameem A., Krovvidi H., “Cerebral physiology,” Contin. Educ. Anaesth. Crit. Care Pain 13(4), 113–118 (2013).10.1093/bjaceaccp/mkt001 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

