ATF3 expression in cardiomyocytes and myofibroblasts following transverse aortic constriction displays distinct phenotypes
- PMID: 33437861
- PMCID: PMC7786009
- DOI: 10.1016/j.ijcha.2020.100706
ATF3 expression in cardiomyocytes and myofibroblasts following transverse aortic constriction displays distinct phenotypes
Abstract
Background: Activating transcription 3 (ATF3) is a member of the basic leucine zipper family of transcription factors. ATF3 is an immediate early gene expressed following various cellular stresses. ATF3 acts through binding to cyclic AMP response elements found in the promoters of key regulatory proteins that determine cell fate. In the heart, multiple cardiac stresses result in chronic ATF3 expression. Transgenic mice with ATF3 expression in cardiomyocytes clearly demonstrate that ATF3 serves a leading role in heart hypertrophy, cardiac fibrosis, cardiac dysfunction and death. In contrast, the use of ATF3 whole body knockout mice resulted non-conclusive results. The heart is composed of various cell types such as cardiomyocytes, fibroblasts, endothelial and immune cells. The question that we addressed in this study is whether ablation of ATF3 in unique cell types in the heart results in diverse cardiac phenotypes.
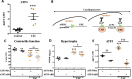
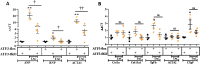
Methods: ATF3-flox mice were crossed with αMHC and Postn specific promoters directing CRE expression and thus ATF3 ablation in cardiomyocytes and myofibroblast cells. Mice were challenged with transverse aortic constriction (TAC) for eight weeks and heart function, ventricle weight, hypertrophic markers, fibrosis markers and ATF3 expression were assessed by qRT-PCR.
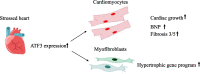
Results: The results of the study show that ATF3 deletion in cardiomyocytes followed by TAC resulted in reduced heart growth and dampened fibrosis response while ATF3 ablation in myofibroblasts displayed a reduced hypertrophic gene program.
Conclusions: TAC-operation results in increased ATF3 expression in both myofibroblasts and cardiomyocytes that promotes a hypertrophic program and fibrotic cardiac growth, respectively.
Keywords: Cardiac remodeling; Cardiomyocytes fibroblasts; Conditional knockout; Heart failure; Hypertrophy; Pressure overload.
© 2020 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
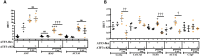

References
-
- Molkentin J.D., Dorn G.W., 2nd Cytoplasmic signaling pathways that regulate cardiac hypertrophy. Annu. Rev. Physiol. 2001;63:391–426. - PubMed
-
- Zou Y., Takano H., Akazawa H., Nagai T., Mizukami M., Komuro I. Molecular and cellular mechanisms of mechanical stress-induced cardiac hypertrophy. Endocr. J. 2002;49:1–13. - PubMed
-
- Bernardo B.C., Weeks K.L., Pretorius L., McMullen J.R. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol. Ther. 2010;128:191–227. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

