Advances in 3D neuronal microphysiological systems: towards a functional nervous system on a chip
- PMID: 33438114
- PMCID: PMC7802613
- DOI: 10.1007/s11626-020-00532-8
Advances in 3D neuronal microphysiological systems: towards a functional nervous system on a chip
Abstract
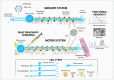
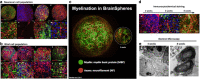
Microphysiological systems (MPS) designed to study the complexities of the peripheral and central nervous systems have made marked improvements over the years and have allowed researchers to assess in two and three dimensions the functional interconnectivity of neuronal tissues. The recent generation of brain organoids has further propelled the field into the nascent recapitulation of structural, functional, and effective connectivities which are found within the native human nervous system. Herein, we will review advances in culture methodologies, focused especially on those of human tissues, which seek to bridge the gap from 2D cultures to hierarchical and defined 3D MPS with the end goal of developing a robust nervous system-on-a-chip platform. These advances have far-reaching implications within basic science, pharmaceutical development, and translational medicine disciplines.
Keywords: 3D culture; Brain organoids; Microengineering; Microphysiological systems; Nerve-on-a-chip; Neuroscience.
Conflict of interest statement
WAA is a paid employee of AxoSim, Inc., New Orleans, LA, USA, which is a for-profit entity commercializing neural microphysiological system. TH and HH are inventors on a patent by Johns Hopkins University, which is licensed to AxoSim, for which they both consult and TH is a shareholder. MJM is an inventor on a patent by Tulane University also licensed by AxoSim, of which he is a co-founder and shareholder. AB declares no conflict of interest.
Figures






References
-
- Anderson WA, Willenberg AR, Bosak AJ, Willenberg BJ, Lambert S. Use of a capillary alginate gel (Capgel) to study the three-dimensional development of sensory nerves reveals the formation of a rudimentary perineurium. J Neurosci Methods. 2018;305:46–53. doi: 10.1016/j.jneumeth.2018.05.003. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

