Clinical Efficacy and Safety of Alirocumab After Acute Coronary Syndrome According to Achieved Level of Low-Density Lipoprotein Cholesterol: A Propensity Score-Matched Analysis of the ODYSSEY OUTCOMES Trial
- PMID: 33438437
- PMCID: PMC7969166
- DOI: 10.1161/CIRCULATIONAHA.120.049447
Clinical Efficacy and Safety of Alirocumab After Acute Coronary Syndrome According to Achieved Level of Low-Density Lipoprotein Cholesterol: A Propensity Score-Matched Analysis of the ODYSSEY OUTCOMES Trial
Abstract
Background: Recent international guidelines have lowered recommended target levels of low-density lipoprotein cholesterol (LDL-C) for patients at very high risk for major adverse cardiovascular events (MACE). However, uncertainty persists whether additional benefit results from achieved LDL-C levels below the conventional targets. Inferences from previous analyses are limited because patients who achieve lower versus higher LDL-C on lipid-lowering therapy differ in other characteristics prognostic for MACE and because few achieved very low LDL-C levels. To overcome these limitations, we performed a propensity score-matching analysis of the ODYSSEY OUTCOMES trial (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab) which compared alirocumab with placebo in 18 924 patients with recent acute coronary syndrome receiving intensive or maximum-tolerated statin treatment.
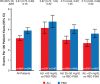
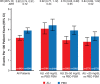
Methods: Patients on alirocumab were classified in prespecified strata of LDL-C achieved at 4 months of treatment: <25 (n=3357), 25 to 50 (n=3692), or >50 mg/dL (n=2197). For each stratum, MACE (coronary heart disease death, nonfatal myocardial infarction, ischemic stroke, or hospitalization for unstable angina) after month 4 was compared in patients receiving placebo with similar baseline characteristics and adherence by using 1:1 propensity score matching.
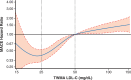
Results: Across achieved LDL-C strata of the alirocumab group, patients differed by baseline LDL-C, lipoprotein(a), use of intensive statin therapy, study medication adherence, and other demographic, medical history, biometric, and laboratory criteria. After propensity score matching, characteristics were similar in corresponding patients of the alirocumab and placebo groups. Treatment hazard ratio, 95% CI, and absolute risk reduction (number per 100 patient-years) for MACE were similar in those with achieved LDL-C <25 mg/dL (hazard ratio, 0.74 [95% CI, 0.62-0.89]; absolute risk reduction, 0.92) or 25 to 50 mg/dL (hazard ratio, 0.74 [95% CI, 0.64-0.87]; absolute risk reduction, 1.05). Patients with achieved LDL-C >50 mg/dL had poorer adherence and derived less benefit (hazard ratio, 0.87 [95% CI, 0.73-1.04]; absolute risk reduction, 0.62). No safety concerns were associated with a limited period of LDL-C levels <15 mg/dL.
Conclusions: After accounting for differences in baseline characteristics and adherence, patients treated with alirocumab who achieved LDL-C levels <25 mg/dL had a reduction in the risk of MACE that was similar to that of patients who achieved LDL-C levels of 25 to 50 mg/dL. Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT01663402.
Keywords: PCSK9 protein, human; acute coronary syndrome; hydroxymethylglutaryl-CoA reductase inhibitors; lipoprotein(a); lipoproteins, LDL.
Figures

References
-
- Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. doi: 10.1126/science.3513311 - PubMed
-
- O’Keefe JH, Jr, Cordain L, Harris WH, Moe RM, Vogel R. Optimal low-density lipoprotein is 50 to 70 mg/dl: lower is better and physiologically normal. J Am Coll Cardiol. 2004;43:2142–2146. doi: 10.1016/j.jacc.2004.03.046 - PubMed
-
- Parker CR, Jr, Carr BR, Simpson ER, MacDonald PC. Decline in the concentration of low-density lipoprotein-cholesterol in human fetal plasma near term. Metabolism. 1983;32:919–923. doi: 10.1016/0026-0495(83)90207-x - PubMed
-
- Forrester JS. Redefining normal low-density lipoprotein cholesterol: a strategy to unseat coronary disease as the nation’s leading killer. J Am Coll Cardiol. 2010;56:630–636. doi: 10.1016/j.jacc.2009.11.090 - PubMed
-
- Mercado CI, Gregg E, Gillespie C, Loustalot F. Trends in lipid profiles and descriptive characteristics of U.S. adults with and without diabetes and cholesterol-lowering medication use-National Health and Nutrition Examination Survey, 2003–2012, United States. PLoS One. 2018;13:e0193756. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

