Design and Synthesis of Tetrahydroisoquinoline Derivatives as Anti-Angiogenesis and Anti-Cancer Agents
- PMID: 33438560
- PMCID: PMC8694809
- DOI: 10.2174/1871520621666210112122913
Design and Synthesis of Tetrahydroisoquinoline Derivatives as Anti-Angiogenesis and Anti-Cancer Agents
Abstract
Aim: The aim of our research work is the synthesis of tetrahydroisoquinoline derivatives as anti-Angiogenesis and anti-cancer agents.
Background: Cancer is the second leading cause of deaths in the United States. The current recovery rate from the advanced treatment for the cancer is excessively low. Therefore, the identification of novel, potent, and less toxic anticancer agents remains a top priority.
Objective: To evaluate anti-angiogenesis and anticancer activities of THIQs on different colorectal cancer cell lines (CRC) viz., Colo320, DLD-1, HCT116, SNU-C1, SW480, and GSK3b in pre-treated viability HCT116. and to carry out molecular docking studies of THIQs.
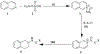
Methods: Twenty synthesized THIQs were screened in the Eli Lilly's Open Innovation Drug Discovery Program and selected twelve compounds for in vitro primary screening in the KRas (Kirsten rat sarcoma)-Wnt SL (Synthetic Lethal) in the basal viability of different colon cancer cell lines. Docking studies of the active THIQs were also performed in our laboratory, targeting the active sites of KRas and VEGF receptors.
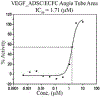
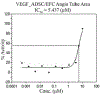
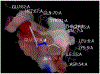
Results: Compound GM-3-18 was found to possess significant activities for KRas inhibition, with IC50 values in the range of 0.9 μM to 10.7 μM, for all colon cancer cell lines. Compound GM-3-121 showed potent anti-angiogenesis activity with IC50 = 1.72 μM. Molecular docking studies showed that the carbonyl oxygen atoms of GM-3-18 and GM-3-121 showed hydrogen bonding interactions with the hydrogen of - OH groups of THR 74 (A).
Conclusion: The results indicated that all the compounds showed moderate to high activity for KRas inhibition. The THIQs bearing the chloro group at the 4-position of the phenyl ring (GM-3-18) exhibited significant KRas inhibition against all colon cancer cell lines.
Keywords: KRas; Tetrahydroisoquinoline derivatives (THIQs); anti-angiogenesis; colon cancer; molecular docking; phenyl ring (GM-3-18)..
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Conflict of interest statement
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All the authors have completed all the Institutional required courses a Responsible Conduct of Research (RCR) and the Conflict of Interest.
CONFLICT OF INTEREST
The authors declare no conflict of interest. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Figures



Similar articles
-
Design, Synthesis and Biological Evaluation of a Novel Series of Thiadiazole- Based Anticancer Agents as Potent Angiogenesis Inhibitors.Anticancer Agents Med Chem. 2021;21(15):2041-2049. doi: 10.2174/1871520621666201231143535. Anticancer Agents Med Chem. 2021. PMID: 33390125
-
Design, synthesis, and biological antitumor evaluation of tetrahydroisoquinoline derivatives.Bioorg Med Chem Lett. 2024 Sep 1;109:129824. doi: 10.1016/j.bmcl.2024.129824. Epub 2024 May 31. Bioorg Med Chem Lett. 2024. PMID: 38823729
-
Design, synthesis and antitumour and anti-angiogenesis evaluation of 22 moscatilin derivatives.Bioorg Med Chem. 2019 Jun 15;27(12):2657-2665. doi: 10.1016/j.bmc.2019.04.027. Epub 2019 Apr 27. Bioorg Med Chem. 2019. PMID: 31047774
-
Substituted tetrahydroisoquinolines: synthesis, characterization, antitumor activity and other biological properties.Eur J Med Chem. 2018 Feb 10;145:51-63. doi: 10.1016/j.ejmech.2017.12.098. Epub 2018 Jan 3. Eur J Med Chem. 2018. PMID: 29324343
-
Design, Synthesis, In Vitro Anti-cancer Activity, ADMET Profile and Molecular Docking of Novel Triazolo[3,4-a]phthalazine Derivatives Targeting VEGFR-2 Enzyme.Anticancer Agents Med Chem. 2018;18(8):1184-1196. doi: 10.2174/1871520618666180412123833. Anticancer Agents Med Chem. 2018. PMID: 29651967
Cited by
-
Morpholine-Substituted Tetrahydroquinoline Derivatives as Potential mTOR Inhibitors: Synthesis, Computational Insights, and Cellular Analysis.Cancers (Basel). 2025 Feb 23;17(5):759. doi: 10.3390/cancers17050759. Cancers (Basel). 2025. PMID: 40075606 Free PMC article.
-
Tetrahydroisoquinoline reduces angiogenesis by interacting myeloma cells with HUVECs mediated by extracellular vesicles.Med Oncol. 2024 Aug 5;41(9):217. doi: 10.1007/s12032-024-02465-8. Med Oncol. 2024. PMID: 39102060
-
Neoadjuvant triple-modality therapy with immune checkpoint blockade, anti-angiogenesis, and chemotherapy enhances pathologic response and survival in locally advanced and metastatic colorectal cancer: a multicenter cohort study.Int J Colorectal Dis. 2025 Jul 9;40(1):154. doi: 10.1007/s00384-025-04945-3. Int J Colorectal Dis. 2025. PMID: 40632233 Free PMC article.
References
-
- Sing IP; Shah P Tetrahydroisoquinolines in therapeutics: a patent review (2010-2015). Expert Opin. Ther. Pat, 2016, •••, 1–20. PMID: 27623022 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

