Sauropus brevipes ethanol extract negatively regulates inflammatory responses in vivo and in vitro by targeting Src, Syk and IRAK1
- PMID: 33439064
- PMCID: PMC7808742
- DOI: 10.1080/13880209.2020.1866024
Sauropus brevipes ethanol extract negatively regulates inflammatory responses in vivo and in vitro by targeting Src, Syk and IRAK1
Abstract
Context: Sauropus brevipes Müll. Arg. (Phyllanthaceae) has been used as an effective ingredient in a decoction for the treatment of diarrhoea. However, there was no report on its modulatory role in inflammation.
Objective: This study investigates anti-inflammatory effect of S. brevipes in various inflammation models.
Materials and methods: The aerial part of S. brevipes was extracted with 95% ethanol to produce Sb-EE. RAW264.7 cells pre-treated with Sb-EE were stimulated by lipopolysaccharide (LPS), and Griess assay and PCR were performed. High-performance liquid chromatography (HPLC) analysis, luciferase assay, Western blotting and kinase assay were employed. C57BL/6 mice (10 mice/group) were orally administered with Sb-EE (200 mg/kg) once a day for five days, and peritonitis was induced by an intraperitoneal injection of LPS (10 mg/kg). ICR mice (four mice/group) were orally administered with Sb-EE (20 or 200 mg/kg) or ranitidine (positive control) twice a day for two days, and EtOH/HCl was orally injected to induce gastritis.
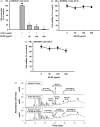
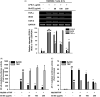
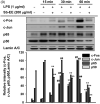
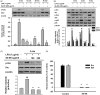
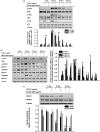
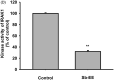
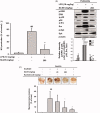
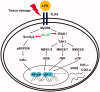
Results: Sb-EE suppressed nitric oxide (NO) release (IC50=34 µg/mL) without cytotoxicity and contained flavonoids (quercetin, luteolin and kaempferol). Sb-EE (200 µg/mL) reduced the mRNA expression of inducible NO synthase (iNOS). Sb-EE blocked the activities of Syk and Src, while inhibiting interleukin-1 receptor associated kinases (IRAK1) by 68%. Similarly, orally administered Sb-EE (200 mg/kg) suppressed NO production by 78% and phosphorylation of Src and Syk in peritonitis mice. Sb-EE also decreased inflammatory lesions in gastritis mice.
Discussion and conclusions: This study demonstrates the inhibitory effect of Sb-EE on the inflammatory response, suggesting that Sb-EE can be developed as a potential anti-inflammatory agent.
Keywords: Natural product medicine; anti-inflammatory remedy; flavonoids; gastritis; peritonitis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Alisma canaliculatum ethanol extract suppresses inflammatory responses in LPS-stimulated macrophages, HCl/EtOH-induced gastritis, and DSS-triggered colitis by targeting Src/Syk and TAK1 activities.J Ethnopharmacol. 2018 Jun 12;219:202-212. doi: 10.1016/j.jep.2018.03.022. Epub 2018 Mar 21. J Ethnopharmacol. 2018. PMID: 29574093
-
Myrsine seguinii ethanolic extract and its active component quercetin inhibit macrophage activation and peritonitis induced by LPS by targeting to Syk/Src/IRAK-1.J Ethnopharmacol. 2014 Feb 12;151(3):1165-1174. doi: 10.1016/j.jep.2013.12.033. Epub 2013 Dec 27. J Ethnopharmacol. 2014. PMID: 24378351
-
Src/Syk/IRAK1-targeted anti-inflammatory action of Torreya nucifera butanol fraction in lipopolysaccharide-activated RAW264.7 cells.J Ethnopharmacol. 2016 Jul 21;188:167-76. doi: 10.1016/j.jep.2016.05.008. Epub 2016 May 10. J Ethnopharmacol. 2016. PMID: 27178629
-
Anti-inflammatory activities of Canarium subulatum Guillaumin methanol extract operate by targeting Src and Syk in the NF-κB pathway.J Ethnopharmacol. 2019 Jun 28;238:111848. doi: 10.1016/j.jep.2019.111848. Epub 2019 Apr 2. J Ethnopharmacol. 2019. PMID: 30951845
-
In vivo and in vitro anti-inflammatory activities of Persicaria chinensis methanolic extract targeting Src/Syk/NF-κB.J Ethnopharmacol. 2015 Jan 15;159:9-16. doi: 10.1016/j.jep.2014.10.064. Epub 2014 Nov 8. J Ethnopharmacol. 2015. PMID: 25446596
Cited by
-
Angiopteris cochinchinensis de Vriese Ameliorates LPS-Induced Acute Lung Injury via Src Inhibition.Plants (Basel). 2022 May 13;11(10):1306. doi: 10.3390/plants11101306. Plants (Basel). 2022. PMID: 35631731 Free PMC article.
-
Effects of Artemisia asiatica ex on Akkermansia muciniphila dominance for modulation of Alzheimer's disease in mice.PLoS One. 2024 Oct 28;19(10):e0312670. doi: 10.1371/journal.pone.0312670. eCollection 2024. PLoS One. 2024. PMID: 39466764 Free PMC article.
-
Hyptis obtusiflora C. Presl ex Benth Methanolic Extract Exhibits Anti-Inflammatory and Anti-Gastritis Activities via Suppressing AKT/NF-κB Pathway.Plants (Basel). 2023 Mar 2;12(5):1146. doi: 10.3390/plants12051146. Plants (Basel). 2023. PMID: 36904006 Free PMC article.
-
Antiphotoaging and Skin-Protective Activities of Ardisia silvestris Ethanol Extract in Human Keratinocytes.Plants (Basel). 2023 Mar 3;12(5):1167. doi: 10.3390/plants12051167. Plants (Basel). 2023. PMID: 36904025 Free PMC article.
-
Immunopharmacological Activities of Luteolin in Chronic Diseases.Int J Mol Sci. 2023 Jan 21;24(3):2136. doi: 10.3390/ijms24032136. Int J Mol Sci. 2023. PMID: 36768462 Free PMC article. Review.
References
-
- Akira S, Takeda K.. 2004. Toll-like receptor signalling. Nat Rev Immunol. 4(7):499–511. - PubMed
-
- Almela L, Sanchez-Munoz B, Fernandez-Lopez JA, Roca MJ, Rabe V.. 2006. Liquid chromatographic–mass spectrometric analysis of phenolics and free radical scavenging activity of rosemary extract from different raw material. J Chromatogr A. 1120(1–2):221–229. - PubMed
-
- Arif T. 2020. Therapeutic potential and traditional uses of Sauropus androgynous: a review. J Pharmacogn Phytochem. 9:2131–2137.
-
- Baeuerle PA, Henkel T.. 1994. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 12:141–179. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
