Antivirals that target the host IMPα/β1-virus interface
- PMID: 33439253
- PMCID: PMC7925013
- DOI: 10.1042/BST20200568
Antivirals that target the host IMPα/β1-virus interface
Abstract
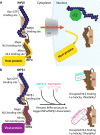
Although transport into the nucleus mediated by the importin (IMP) α/β1-heterodimer is central to viral infection, small molecule inhibitors of IMPα/β1-dependent nuclear import have only been described and shown to have antiviral activity in the last decade. Their robust antiviral activity is due to the strong reliance of many different viruses, including RNA viruses such as human immunodeficiency virus-1 (HIV-1), dengue (DENV), and Zika (ZIKV), on the IMPα/β1-virus interface. High-throughput compound screens have identified many agents that specifically target this interface. Of these, agents targeting IMPα/β1 directly include the FDA-approved macrocyclic lactone ivermectin, which has documented broad-spectrum activity against a whole range of viruses, including HIV-1, DENV1-4, ZIKV, West Nile virus (WNV), Venezuelan equine encephalitis virus, chikungunya, and most recently, SARS-CoV-2 (COVID-19). Ivermectin has thus far been tested in Phase III human clinical trials for DENV, while there are currently close to 80 trials in progress worldwide for SARS-CoV-2; preliminary results for randomised clinical trials (RCTs) as well as observational/retrospective studies are consistent with ivermectin affording clinical benefit. Agents that target the viral component of the IMPα/β1-virus interface include N-(4-hydroxyphenyl) retinamide (4-HPR), which specifically targets DENV/ZIKV/WNV non-structural protein 5 (NS5). 4-HPR has been shown to be a potent inhibitor of infection by DENV1-4, including in an antibody-dependent enhanced animal challenge model, as well as ZIKV, with Phase II clinical challenge trials planned. The results from rigorous RCTs will help determine the therapeutic potential of the IMPα/β1-virus interface as a target for antiviral development.
Keywords: SARS-CoV-2; Zika virus; antiviral agents; dengue virus; importin; ivermectin.
© 2021 The Author(s).
Conflict of interest statement
D.A.J. is an inventor on a patent application for the use of ivermectin for coronavirus infection, and a patent on the use of 4-HPR in flavivirus infection. A.J.M. declares no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

