Elevated plasma IL-6 and CRP levels are associated with adverse clinical outcomes and death in critically ill SARS-CoV-2 patients: inflammatory response of SARS-CoV-2 patients
- PMID: 33439360
- PMCID: PMC7804215
- DOI: 10.1186/s13613-020-00798-x
Elevated plasma IL-6 and CRP levels are associated with adverse clinical outcomes and death in critically ill SARS-CoV-2 patients: inflammatory response of SARS-CoV-2 patients
Erratum in
-
Correction to: Elevated plasma IL-6 and CRP levels are associated with adverse clinical outcomes and death in critically ill SARS-CoV-2 patients: infammatory response of SARS-CoV-2 patients.Ann Intensive Care. 2021 Jun 9;11(1):93. doi: 10.1186/s13613-021-00879-5. Ann Intensive Care. 2021. PMID: 34106340 Free PMC article. No abstract available.
Abstract
Background: SARS coronavirus 2 (SARS-CoV-2) is responsible for high morbidity and mortality worldwide, mostly due to the exacerbated inflammatory response observed in critically ill patients. However, little is known about the kinetics of the systemic immune response and its association with survival in SARS-CoV-2+ patients admitted in ICU. We aimed to compare the immuno-inflammatory features according to organ failure severity and in-ICU mortality.
Methods: Six-week multicentre study (N = 3) including SARS-CoV-2+ patients admitted in ICU. Analysis of plasma biomarkers at days 0 and 3-4 according to organ failure worsening (increase in SOFA score) and 60-day mortality.
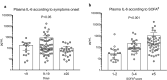
Results: 101 patients were included. Patients had severe respiratory diseases with PaO2/FiO2 of 155 [111-251] mmHg), SAPS II of 37 [31-45] and SOFA score of 4 [3-7]. Eighty-three patients (83%) required endotracheal intubation/mechanical ventilation and among them, 64% were treated with prone position. IL-1β was barely detectable. Baseline IL-6 levels positively correlated with organ failure severity. Baseline IL-6 and CRP levels were significantly higher in patients in the worsening group than in the non-worsening group (278 [70-622] vs. 71 [29-153] pg/mL, P < 0.01; and 178 [100-295] vs. 100 [37-213] mg/L, P < 0.05, respectively). Baseline IL-6 and CRP levels were significantly higher in non-survivors compared to survivors but fibrinogen levels and lymphocyte counts were not different between groups. After adjustment on SOFA score and time from symptom onset to first dosage, IL-6 and CRP remained significantly associated with mortality. IL-6 changes between Day 0 and Day 3-4 were not different according to the outcome. A contrario, kinetics of CRP and lymphocyte count were different between survivors and non-survivors.
Conclusions: In SARS-CoV-2+ patients admitted in ICU, a systemic pro-inflammatory signature was associated with clinical worsening and 60-day mortality.
Keywords: Covid-19; Cytokine; Inflammation; Outcome; SARS-CoV-2.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

