The Xyrem® (Sodium Oxybate) Risk Evaluation and Mitigation Strategy (REMS) Program in the USA: Results From 2016 to 2017
- PMID: 33439474
- PMCID: PMC7984153
- DOI: 10.1007/s40801-020-00223-6
The Xyrem® (Sodium Oxybate) Risk Evaluation and Mitigation Strategy (REMS) Program in the USA: Results From 2016 to 2017
Abstract
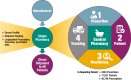
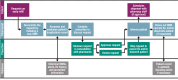
Background: Sodium oxybate, which is approved for the treatment of cataplexy or excessive daytime sleepiness in patients with narcolepsy, is available in the USA only through the restricted-distribution Xyrem® Risk Evaluation and Mitigation Strategy Program (Xyrem REMS Program, XRP). The XRP requires prescriber enrollment and certification, patient enrollment, and prescriber attestation of patient counseling. Sodium oxybate is dispensed only by the certified pharmacy. After pharmacist/patient counseling, sodium oxybate is shipped only to enrolled patients, with documentation of safe use. Documentation of enrollments, prescriptions, counseling, shipments, and adverse events in a central database, and risk management reporting of any suspicion of abuse, misuse, or diversion, ensure provider notification and facilitate monitoring.
Objective: This analysis reports data from the XRP regarding assessment of the risks of serious adverse outcomes that may result from inappropriate prescribing, abuse, misuse, and diversion.
Methods: Data collected from December 2016 to December 2017 were analyzed.
Results: Prescriptions were from enrolled prescribers (n = 4524); 17,037 patients received one or more shipment of sodium oxybate. No patients were shipped sodium oxybate under more than one name/identifier or after being disenrolled; no individual patient had overlapping active prescriptions. Sodium oxybate was dispensed in 146,426 shipments containing 375,173 bottles; of those, 13 shipments (0.009%) and 26 bottles (0.007%) were lost in delivery and not recovered. Notifications regarding potential abuse (n = 31), misuse (n = 343), or diversion (n = 22) were discussed with prescribers. Most patients and prescribers were aware of the main safety risks of sodium oxybate.
Conclusions: The XRP maintains controlled access to sodium oxybate; additional prescriber education on safety risks may be warranted.
Conflict of interest statement
Michael J. Strunc has received research funding from Jazz Pharmaceuticals, has received honoraria for grand rounds, and has served on the speakers’ bureau for Jazz Pharmaceuticals and has provided expert testimony. Jed Black is a part-time employee of Jazz Pharmaceuticals and shareholder of Jazz Pharmaceuticals plc. Prasheel Lillaney, Sherice Mills, and Shay Bujanover are employees of Jazz Pharmaceuticals who, in the course of their employment, have received stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals plc. Judi Profant is a former employee of Jazz Pharmaceuticals who, in the course of her employment, received stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals plc. Michael J. Thorpy has received research/grant support and consultancy fees from Jazz Pharmaceuticals, Axsome Therapeutics, Balance Therapeutics, Avadel Pharmaceuticals, Takeda Pharmaceuticals, Harmony Biosciences, and Suven Life Sciences.
Figures
References
-
- American Academy of Sleep Medicine . International classification of sleep disorders. 3. Darien: American Academy of Sleep Medicine; 2014.
LinkOut - more resources
Full Text Sources
Other Literature Sources