Updated Overall Survival and PD-L1 Subgroup Analysis of Patients With Extensive-Stage Small-Cell Lung Cancer Treated With Atezolizumab, Carboplatin, and Etoposide (IMpower133)
- PMID: 33439693
- PMCID: PMC8078320
- DOI: 10.1200/JCO.20.01055
Updated Overall Survival and PD-L1 Subgroup Analysis of Patients With Extensive-Stage Small-Cell Lung Cancer Treated With Atezolizumab, Carboplatin, and Etoposide (IMpower133)
Abstract
Purpose: IMpower133 (ClinicalTrials.gov identifier: NCT02763579), a randomized, double-blind, phase I/III study, demonstrated that adding atezolizumab (anti-programmed death-ligand 1 [PD-L1]) to carboplatin plus etoposide (CP/ET) for first-line (1L) treatment of extensive-stage small-cell lung cancer (ES-SCLC) resulted in significant improvement in overall survival (OS) and progression-free survival (PFS) versus placebo plus CP/ET. Updated OS, disease progression patterns, safety, and exploratory biomarkers (PD-L1, blood-based tumor mutational burden [bTMB]) are reported.
Patients and methods: Patients with untreated ES-SCLC were randomly assigned 1:1 to receive four 21-day cycles of CP (area under the curve 5 mg per mL/min intravenously [IV], day 1) plus ET (100 mg/m2 IV, days 1-3) with atezolizumab (1,200 mg IV, day 1) or placebo, and then maintenance atezolizumab or placebo until unacceptable toxicity, disease progression, or loss of clinical benefit. Tumor specimens were collected; PD-L1 testing was not required for enrollment. The two primary end points, investigator-assessed PFS and OS, were statistically significant at the interim analysis. Updated OS and PFS and exploratory biomarker analyses were conducted.
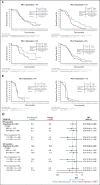
Results: Patients received atezolizumab plus CP/ET (n = 201) or placebo plus CP/ET (n = 202). At the updated analysis, median follow-up for OS was 22.9 months; 302 deaths had occurred. Median OS was 12.3 and 10.3 months with atezolizumab plus CP/ET and placebo plus CP/ET, respectively (hazard ratio, 0.76; 95% CI, 0.60 to 0.95; descriptive P = .0154). At 18 months, 34.0% and 21.0% of patients were alive in atezolizumab plus CP/ET and placebo plus CP/ET arms, respectively. Patients derived benefit from the addition of atezolizumab, regardless of PD-L1 immunohistochemistry or bTMB status.
Conclusion: Adding atezolizumab to CP/ET as 1L treatment for ES-SCLC continued to demonstrate improved OS and a tolerable safety profile at the updated analysis, confirming the regimen as a new standard of care. Exploratory analyses demonstrated treatment benefit independent of biomarker status.
Figures


References
-
- Bernhardt EB, Jalal SI: Small cell lung cancer. Cancer Treat Res 170:301-322, 2016 - PubMed
-
- National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology: Small Cell Lung Cancer (Version 1.2019), 2019
-
- Armstrong SA, Liu SV: Dashing decades of defeat: Long anticipated advances in the first-line treatment of extensive-stage small cell lung cancer. Curr Oncol Reports 22:20, 2020 - PubMed
-
- Horn L Mansfield AS Szczęsna A, et al. : First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 379:2220-2229, 2018 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

