College of American Pathologists Cancer Protocols: From Optimizing Cancer Patient Care to Facilitating Interoperable Reporting and Downstream Data Use
- PMID: 33439728
- PMCID: PMC8140812
- DOI: 10.1200/CCI.20.00104
College of American Pathologists Cancer Protocols: From Optimizing Cancer Patient Care to Facilitating Interoperable Reporting and Downstream Data Use
Abstract
The College of American Pathologists Cancer Protocols have offered guidance to pathologists for standard cancer pathology reporting for more than 35 years. The adoption of computer readable versions of these protocols by electronic health record and laboratory information system (LIS) vendors has provided a mechanism for pathologists to report within their LIS workflow, in addition to enabling standardized structured data capture and reporting to downstream consumers of these data such as the cancer surveillance community. This paper reviews the history of the Cancer Protocols and electronic Cancer Checklists, outlines the current use of these critically important cancer case reporting tools, and examines future directions, including plans to help improve the integration of the Cancer Protocols into clinical, public health, research, and other workflows.
Figures



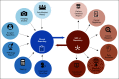
References
-
- Hutter RV, Henson DE: The pathologist as a consultant in cancer patient management: A patterns of care study in pathology. Int J Radiat Oncol Biol Phys 10:45-47, 1984(suppl 1) - PubMed
-
- Hammond ME, Compton CC: Protocols for the examination of tumors of diverse sites: Introduction. Cancer Committee of the College of American Pathologists. Arch Pathol Lab Med 124:13-16, 2000 - PubMed
-
- Hutter RVP: Guidelines for data to be included in consultation reports on breast cancer, bladder cancer, and Hodgkin’s disease. Pathologist 40:18-23, 1986
-
- Grignon DG, Hammond E: College of American Pathologists conference XXVI on clinical relevance of prognostic markers in solid tumors: Report of the Prostate Cancer Working Group. Arch Pathol Lab Med 119:1122-1126, 1995 - PubMed
-
- Farrow G, Amin MB: Protocol for the examination of specimens from patients with carcinomas of renal tubular origin, exclusive of Wilms tumor and tumors of urothelial origin: A basis for checklists: Cancer Committee, College of American Pathologists. Arch Pathol Lab Med 123:23–27, 1999 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

