Plasma proteins associated with cardiovascular death in patients with chronic coronary heart disease: A retrospective study
- PMID: 33439866
- PMCID: PMC7817029
- DOI: 10.1371/journal.pmed.1003513
Plasma proteins associated with cardiovascular death in patients with chronic coronary heart disease: A retrospective study
Abstract
Background: Circulating biomarkers are associated with the development of coronary heart disease (CHD) and its complications by reflecting pathophysiological pathways and/or organ dysfunction. We explored the associations between 157 cardiovascular (CV) and inflammatory biomarkers and CV death using proximity extension assays (PEA) in patients with chronic CHD.
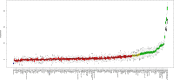
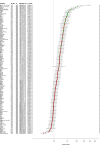
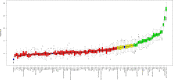
Methods and findings: The derivation cohort consisted of 605 cases with CV death and 2,788 randomly selected non-cases during 3-5 years follow-up included in the STabilization of Atherosclerotic plaque By Initiation of darapLadIb TherapY (STABILITY) trial between 2008 and 2010. The replication cohort consisted of 245 cases and 1,042 non-cases during 12 years follow-up included in the Ludwigshafen Risk and Cardiovascular Health (LURIC) study between 1997 and 2000. Biomarker levels were measured with conventional immunoassays and/or with the OLINK PEA panels CVD I and Inflammation. Associations with CV death were evaluated by Random Survival Forest (RF) and Cox regression analyses. Both cohorts had the same median age (65 years) and 20% smokers, while there were slight differences in male sex (82% and 76%), hypertension (70% and 78%), and diabetes (39% and 30%) in the respective STABILITY and LURIC cohorts. The analyses identified 18 biomarkers with confirmed independent association with CV death by Boruta analyses and statistical significance (all p < 0.0001) by Cox regression when adjusted for clinical characteristics in both cohorts. Most prognostic information was carried by N-terminal prohormone of brain natriuretic peptide (NTproBNP), hazard ratio (HR for 1 standard deviation [SD] increase of the log scale of the distribution of the biomarker in the replication cohort) 2.079 (95% confidence interval [CI] 1.799-2.402), and high-sensitivity troponin T (cTnT-hs) HR 1.715 (95% CI 1.491-1.973). The other proteins with independent associations were growth differentiation factor 15 (GDF-15) HR 1.728 (95% CI 1.527-1.955), transmembrane immunoglobulin and mucin domain protein (TIM-1) HR 1.555 (95% CI 1.362-1.775), renin HR 1.501 (95% CI 1.305-1.727), osteoprotegerin (OPG) HR 1.488 (95% CI 1.297-1.708), soluble suppression of tumorigenesis 2 protein (sST2) HR 1.478 (95% CI 1.307-1.672), cystatin-C (Cys-C) HR 1.370 (95% CI 1.243-1.510), tumor necrosis factor-related apoptosis-inducing ligand receptor 2 (TRAIL-R2) HR 1.205 (95% CI 1.131-1.285), carbohydrate antigen 125 (CA-125) HR 1.347 (95% CI 1.226-1.479), brain natriuretic peptide (BNP) HR 1.399 (95% CI 1.255-1.561), interleukin 6 (IL-6) HR 1.478 (95% CI 1.316-1.659), hepatocyte growth factor (HGF) HR 1.259 (95% CI 1.134-1.396), spondin-1 HR 1.295 (95% CI 1.156-1.450), fibroblast growth factor 23 (FGF-23) HR 1.349 (95% CI 1.237-1.472), chitinase-3 like protein 1 (CHI3L1) HR 1.284 (95% CI 1.129-1.461), tumor necrosis factor receptor 1 (TNF-R1) HR 1.486 (95% CI 1.307-1.689), and adrenomedullin (AM) HR 1.750 (95% CI 1.490-2.056). The study is limited by the differences in design, size, and length of follow-up of the 2 studies and the lack of results from coronary angiograms and follow-up of nonfatal events.
Conclusions: Profiles of levels of multiple plasma proteins might be useful for the identification of different pathophysiological pathways associated with an increased risk of CV death in patients with chronic CHD.
Trial registration: ClinicalTrials.gov NCT00799903.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: LW reports institutional research grants from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer, GlaxoSmithKline, Merck & Co, Roche Diagnostics; consulting fees from Abbott; holds two patents involving GDF-15, both licensed to Roche Diagnostics. NE and MO report institutional research grants from GlaxoSmithKline. EH reports institutional research grants from AstraZeneca, Amgen, GlaxoSmithKline, Sanofi; consulting fees from Amgen, NovoNordisk, Sanofi; speaker fees from AstraZeneca, Amgen, Boehringer Ingelheim, NovoNordisk, Sanofi; principal investigator fees paid to institution from CSL Behring, Dalcor, Regeneron, Sanofi, The Medicines Company. WK reports research grants from Roche Diagnostics, Beckmann, Singulex, Abbott; other research support from European Research Agency (ERA-CVD); honoraria from Novartis, Pfizer, Sanofi, AstraZeneca, Amgen; expert witness fees from Novartis; consultant/advisory board fees from Novartis, Pfizer, DalCor, Sanofi, Kowa, Amgen. CH reports Honoraria from Pfizer; consultant/advisory board fees from AstraZeneca, Bayer, Boehringer Ingelheim. MEK reports consulting/speaker fees from Bayer. WM reports grants and personal fees from Siemens Diagnostics, Abbott Diagnostics, AMGEN, Astrazeneca, Sanofi, BASF, Numares AG, employment from SYNLAB Holding Deutschland GmbH. RAHS reports grants and non-financial support from GlaxoSmithKline. HDW reports research grants from Sanofi-Aventis, Dalcor Pharma, NIH, Eisai, Omthera Pharmaceuticals; other support from SAHMRI, ESC, AHA, Sanofi/Regeneron. AS reports institutional research grants from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer, Roche Diagnostics, GlaxoSmithKline; consulting fees from Olink Proteomics. TBG and MÅ have nothing to declare.
Figures

References
-
- Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease: Executive Summary: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:2564–603. 10.1016/j.jacc.2012.07.012 - DOI - PubMed
-
- Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–77. - PubMed
-
- Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267–315. 10.1093/eurheartj/ehv320 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

