Heparin solution in the prevention of occlusions in Hickman® catheters a randomized clinical trial
- PMID: 33439945
- PMCID: PMC7798395
- DOI: 10.1590/1518-8345.3310.3385
Heparin solution in the prevention of occlusions in Hickman® catheters a randomized clinical trial
Abstract
Objective: to evaluate the effectiveness of the 50 IU/mL heparin solution compared to the 0.9% isotonic saline solution in preventing occlusion of the double lumen Hickman® catheter, 7 and 9 French, in patients undergoing hematopoietic stem cell transplantation.
Method: a triple-blind randomized clinical trial. 17 double-lumen catheters (heparin group: n=7 and 0.9% isotonic saline group: n=10) were analyzed in which the two catheter routes were evaluated separately, totaling 34 lumens. The outcome variables were occlusion without reflux and complete occlusion. Descriptive analyses were performed using the Chi-square test and, of survival, according to the Kaplan-Meier test.
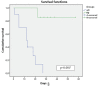
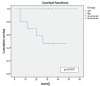
Results: the mean number of days until the occlusion outcome was 52 in the heparin group and 13.46 in the 0.9% isotonic saline group in the white catheter route (p<0.001). In the red route, the mean follow-up days in the heparin group were 35.29, with no occlusion and 22.30 in the 0.9% isotonic saline group until the first occlusion (p=0.030).
Conclusion: blocking with 50 IU/mL heparin solution is more effective than 0.9% isotonic saline in preventing occlusion of the Hickman® catheter. Brazilian Registry of Clinical Trials: RBR-3ht499.
Objetivo:: avaliar a efetividade da solução de heparina 50 UI/mL comparada à solução salina isotônica 0,9% na prevenção de oclusão do Cateter de Hickman® duplo lúmen, 7 e 9 french, em pacientes submetidos ao transplante de células-tronco hematopoéticas.
Método:: ensaio clínico randomizado triplo cego. Foram analisados 17 cateteres duplo lúmen (grupo heparina n=7 e grupo solução salina isotônica 0,9% n=10) nos quais as duas vias do cateter foram avaliadas separadamente, totalizando 34 lúmens. As variáveis de desfecho foram oclusão sem refluxo e oclusão completa. As análises descritivas foram realizadas mediante o teste Qui-quadrado e, de sobrevida, sob o teste de Kaplan-Meier.
Resultados:: a média de dias até o desfecho oclusão foi de 52 no grupo heparina e de 13,46 no grupo solução salina isotônica 0,9% na via branca do cateter (p<0,001). Na via vermelha, a média de dias de acompanhamento do grupo heparina foi de 35,29, sem ocorrência de oclusão, e de 22,30 no grupo solução salina isotônica 0,9% até a primeira oclusão (p=0,030).
Conclusão:: o bloqueio com solução de heparina 50 UI/mL é mais efetivo em relação à solução salina isotônica 0,9% na prevenção da oclusão do Cateter de Hickman®. Registro Brasileiro de Ensaios Clínicos: RBR-3ht499.
Objetivo:: evaluar la eficacia de la solución de heparina 50 UI/mL comparada con la solución salina isotónica al 0,9% para prevenir oclusiones en catéteres de Hickman® doble lumen, 7 y 9 French, en pacientes sometidos a trasplante de células madre hematopoyéticas.
Método:: ensayo clínico aleatorizado triple ciego. Se analizaron 17 catéteres de doble lumen (grupo de heparina: n=7 y grupo de solución salina isotónica al 0,9%: n=10) en los que se evaluaron por separado las dos vías del catéter, totalizando 34 lúmenes. Las variables de resultado fueron oclusión sin reflujo y oclusión completa. Los análisis descriptivos se realizaron mediante el test de Chi-cuadrado y, los de sobrevida, con el test de Kaplan-Meier.
Resultados:: la media de días hasta el resultado de oclusión fue de 52 en el grupo de heparina y de 13,46 en el grupo de la solución salina isotónica al 0,9% en la vía blanca del catéter (p<0,001). En la vía roja, la media de días de seguimiento del grupo de heparina fue de 35,29 sin oclusión y de 22,30 en el del grupo solución salina isotónica al 0,9% hasta la primera oclusión (p=0,030).
Conclusión:: el bloqueo con solución de heparina 50 UI/mL es más eficaz en relación con la solución salina isotónica al 0,9% para prevenir oclusiones en catéteres de Hickman®. Registro Brasileño de Ensayos Clínicos: RBR 3ht499.
Figures


References
-
- Canadian Vascular Access Association Occlusion management guideline for central venous access devices. [Jul 15, 2016];Vascular Access. 2013 7(1):1–36. [Internet] Available from: http://www.improvepicc.com/uploads/5/6/5/0/56503399/omg_2013_final_revis....
-
- Danski MTR, Silva SR, Pontes L, Pedrolo E. Educational action for standardization in the management of Hickman(r) Catheters. [Jun 27, 2019];Cogitare Enferm. 2018 23(3):e54488. [Internet] Available from: https://revistas.ufpr.br/cogitare/article/view/54488/pdf_1.
-
- Ministério da Saúde (BR) Agência Nacional de Vigilância Sanitária . Medidas de prevenção de infecção relacionada à assistência à saúde. Brasília: ANVISA; 2017. [25 jul 2016]. Disponível em: http://portal.anvisa.gov.br/documents/33852/3507912/Caderno+4+-+Medidas+....
-
- Instituto Nacional do Câncer José Alencar Gomes da Silva . Perguntas frequentes: transplante de medula óssea. Rio de Janeiro: INCA; 2017. [15 dez 2017]. [Internet] Disponível em: https://www.inca.gov.br/perguntas-frequentes/transplante-de-medula-ossea.
-
- Schiffer CA, Mangu PB, Wade JC, Camp-Sorrell D, Cope DG, El-Rayes BF, et al. Central venous catheter care for the patient with cancer: American Society of Clinical Oncology clinical practice guideline. [Jul 25, 2016];J Clin Oncol. 2013 31(10):1357–1370. [Internet] Available from: https://www.ncbi.nlm.nih.gov/pubmed/23460705. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

