Efficient deletion of multiple circle RNA loci by CRISPR-Cas9 reveals Os06circ02797 as a putative sponge for OsMIR408 in rice
- PMID: 33440058
- PMCID: PMC8196656
- DOI: 10.1111/pbi.13544
Efficient deletion of multiple circle RNA loci by CRISPR-Cas9 reveals Os06circ02797 as a putative sponge for OsMIR408 in rice
Abstract
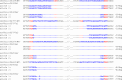
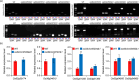
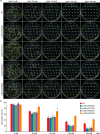
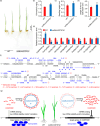
CRISPR-Cas9 is an emerging genome editing tool for reverse genetics in plants. However, its application for functional study of non-coding RNAs in plants is still at its infancy. Despite being a major class of non-coding RNAs, the biological roles of circle RNAs (circRNAs) remain largely unknown in plants. Previous plant circRNA studies have focused on identification and annotation of putative circRNAs, with their functions largely uninvestigated by genetic approaches. Here, we applied a multiplexed CRISPR-Cas9 strategy to efficiently acquire individual null mutants for four circRNAs in rice. We showed each of these rice circRNA loci (Os02circ25329, Os06circ02797, Os03circ00204 and Os05circ02465) can be deleted at 10% or higher efficiency in both protoplasts and stable transgenic T0 lines. Such high efficiency deletion enabled the generation of circRNA null allele plants without the CRISPR-Cas9 transgene in the T1 generation. Characterization of the mutants reveals these circRNAs' participation in salt stress response during seed germination and in particular the Os05circ02465 null mutant showed high salt tolerance. Notably, the seedlings of the Os06circ02797 mutant showed rapid growth phenotype after seed germination with the seedlings containing higher chlorophyll A/B content. Further molecular and computational analyses suggested a circRNA-miRNA-mRNA regulatory network where Os06circ02797 functions to bind and sequester OsMIR408, an important and conserved microRNA in plants. This study not only presents genetic evidence for the first time in plants that certain circRNAs may serve as sponges to negatively regulate miRNAs, a phenomenon previously demonstrated in mammalian cells, but also provides important insights for improving agronomic traits through gene editing of circRNA loci in crops.
Keywords: CRISPR-Cas9; circle RNA; large deletion; microRNA sponge; rice.
© 2021 The Authors. Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Axtell, M.J. and Bowman, J.L. (2008) Evolution of plant microRNAs and their targets. Trends Plant Sci. 13, 343–349. - PubMed
-
- Chen, L.L. (2016) The biogenesis and emerging roles of circular RNAs. Nat. Rev. Mol. Cell Biol. 17, 205–211. - PubMed
-
- Chen, L.L. (2020) The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat. Rev. Mol. Cell Biol. 21, 475–490. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

