Interim Results of a Phase 1-2a Trial of Ad26.COV2.S Covid-19 Vaccine
- PMID: 33440088
- PMCID: PMC7821985
- DOI: 10.1056/NEJMoa2034201
Interim Results of a Phase 1-2a Trial of Ad26.COV2.S Covid-19 Vaccine
Abstract
Background: Efficacious vaccines are urgently needed to contain the ongoing coronavirus disease 2019 (Covid-19) pandemic of infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). A candidate vaccine, Ad26.COV2.S, is a recombinant, replication-incompetent adenovirus serotype 26 (Ad26) vector encoding a full-length and stabilized SARS-CoV-2 spike protein.
Methods: In this multicenter, placebo-controlled, phase 1-2a trial, we randomly assigned healthy adults between the ages of 18 and 55 years (cohort 1) and those 65 years of age or older (cohort 3) to receive the Ad26.COV2.S vaccine at a dose of 5×1010 viral particles (low dose) or 1×1011 viral particles (high dose) per milliliter or placebo in a single-dose or two-dose schedule. Longer-term data comparing a single-dose regimen with a two-dose regimen are being collected in cohort 2; those results are not reported here. The primary end points were the safety and reactogenicity of each dose schedule.
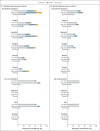
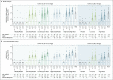
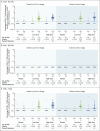
Results: After the administration of the first vaccine dose in 805 participants in cohorts 1 and 3 and after the second dose in cohort 1, the most frequent solicited adverse events were fatigue, headache, myalgia, and injection-site pain. The most frequent systemic adverse event was fever. Systemic adverse events were less common in cohort 3 than in cohort 1 and in those who received the low vaccine dose than in those who received the high dose. Reactogenicity was lower after the second dose. Neutralizing-antibody titers against wild-type virus were detected in 90% or more of all participants on day 29 after the first vaccine dose (geometric mean titer [GMT], 212 to 354), regardless of vaccine dose or age group, and reached 96% by day 57 with a further increase in titers (GMT, 288 to 488) in cohort 1a. Titers remained stable until at least day 71. A second dose provided an increase in the titer by a factor of 2.6 to 2.9 (GMT, 827 to 1266). Spike-binding antibody responses were similar to neutralizing-antibody responses. On day 15, CD4+ T-cell responses were detected in 76 to 83% of the participants in cohort 1 and in 60 to 67% of those in cohort 3, with a clear skewing toward type 1 helper T cells. CD8+ T-cell responses were robust overall but lower in cohort 3.
Conclusions: The safety and immunogenicity profiles of Ad26.COV2.S support further development of this vaccine candidate. (Funded by Johnson & Johnson and the Biomedical Advanced Research and Development Authority of the Department of Health and Human Services; COV1001 ClinicalTrials.gov number, NCT04436276.).
Copyright © 2021 Massachusetts Medical Society.
Figures
References
-
- World Health Organization. Pneumonia of unknown cause — China. January 5, 2020. (https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-ch...).
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
