Dynamic Shifts in the Composition of Resident and Recruited Macrophages Influence Tissue Remodeling in NASH
- PMID: 33440159
- PMCID: PMC7877246
- DOI: 10.1016/j.celrep.2020.108626
Dynamic Shifts in the Composition of Resident and Recruited Macrophages Influence Tissue Remodeling in NASH
Erratum in
-
Dynamic Shifts in the Composition of Resident and Recruited Macrophages Influence Tissue Remodeling in NASH.Cell Rep. 2022 Nov 15;41(7):111660. doi: 10.1016/j.celrep.2022.111660. Cell Rep. 2022. PMID: 36384118 No abstract available.
Abstract
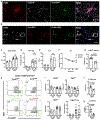
Macrophage-mediated inflammation is critical in the pathogenesis of non-alcoholic steatohepatitis (NASH). Here, we describe that, with high-fat, high-sucrose-diet feeding, mature TIM4pos Kupffer cells (KCs) decrease in number, while monocyte-derived Tim4neg macrophages accumulate. In concert, monocyte-derived infiltrating macrophages enter the liver and consist of a transitional subset that expresses Cx3cr1/Ccr2 and a second subset characterized by expression of Trem2, Cd63, Cd9, and Gpmnb; markers ascribed to lipid-associated macrophages (LAMs). The Cx3cr1/Ccr2-expressing macrophages, referred to as C-LAMs, localize to macrophage aggregates and hepatic crown-like structures (hCLSs) in the steatotic liver. In C-motif chemokine receptor 2 (Ccr2)-deficient mice, C-LAMs fail to appear in the liver, and this prevents hCLS formation, reduces LAM numbers, and increases liver fibrosis. Taken together, our data reveal dynamic changes in liver macrophage subsets during the pathogenesis of NASH and link these shifts to pathologic tissue remodeling.
Keywords: CCR2; Cx3cr1; Kupffer cells; crown-like structures; diabetes; fibrosis; inflammation; lipid-associated macrophages; liver.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests A.E.F. is co-inventor on pending and issued patents filed by the Cleveland Clinic and UCSD that refer to the use of biomarkers and therapies in inflammatory and fibrotic disorders. Scientific Founder: Jecure Therapeutics, Elgia Therapeutics. Consultant/Advisory Board: Gilead, GSK, Merck, Ferring Pharmaceutical, Centurion BioPharma, Oppilan Pharma. The remaining authors have no interests to declare.
Figures






References
-
- Bajpai G, Bredemeyer A, Li W, Zaitsev K, Koenig AL, Lokshina I, Mohan J, Ivey B, Hsiao HM, Weinheimer C, et al. (2019). Tissue Resident CCR2− and CCR2+ Cardiac Macrophages Differentially Orchestrate Monocyte Recruitment and Fate Specification Following Myocardial Injury. Circ. Res 124, 263–278. - PMC - PubMed
-
- Beattie L, Sawtell A, Mann J, Frame TCM, Teal B, de Labastida Rivera F, Brown N, Walwyn-Brown K, Moore JWJ, MacDonald S, et al. (2016). Bone marrow-derived and resident liver macrophages display unique transcriptomic signatures but similar biological functions. J. Hepatol 65, 758–768. - PMC - PubMed
-
- Bonnardel J, T’Jonck W, Gaublomme D, Browaeys R, Scott CL, Martens L, Vanneste B, De Prijck S, Nedospasov SA, Kremer A, et al. (2019). Stellate Cells, Hepatocytes, and Endothelial Cells Imprint the Kupffer Cell Identity on Monocytes Colonizing the Liver Macrophage Niche. Immunity 51, 638–654. - PMC - PubMed
-
- Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, and Bacon BR (1999). Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am. J. Gastroenterol 94, 2467–2474. - PubMed
-
- Devisscher L, Scott CL, Lefere S, Raevens S, Bogaerts E, Paridaens A, Verhelst X, Geerts A, Guilliams M, and Van Vlierberghe H (2017). Non-alcoholic steatohepatitis induces transient changes within the liver macrophage pool. Cell. Immunol 322, 74–83. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

