Role of dietary fiber in the recovery of the human gut microbiome and its metabolome
- PMID: 33440171
- PMCID: PMC8022197
- DOI: 10.1016/j.chom.2020.12.012
Role of dietary fiber in the recovery of the human gut microbiome and its metabolome
Abstract
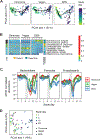
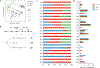
Gut microbiota metabolites may be important for host health, yet few studies investigate the correlation between human gut microbiome and production of fecal metabolites and their impact on the plasma metabolome. Since gut microbiota metabolites are influenced by diet, we performed a longitudinal analysis of the impact of three divergent diets, vegan, omnivore, and a synthetic enteral nutrition (EEN) diet lacking fiber, on the human gut microbiome and its metabolome, including after a microbiota depletion intervention. Omnivore and vegan, but not EEN, diets altered fecal amino acid levels by supporting the growth of Firmicutes capable of amino acid metabolism. This correlated with relative abundance of a sizable number of fecal amino acid metabolites, some not previously associated with the gut microbiota. The effect on the plasma metabolome, in contrast, were modest. The impact of diet, particularly fiber, on the human microbiome influences broad classes of metabolites that may modify health.
Keywords: dietary fiber, vegan, omnivore, amino acid; enteral nutrition; metabolome; microbiome.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests J.B. is on scientific advisory boards of Janssen Research and Prolacta Bioscience. M.A.F is a co-founder of Federation Bio. J.D.L. has received honorarium from Nestle Health Science for consulting and for participation in medical education events. F.D.R., J.D.L., and G.D.W. are co-inventors on patent no. US 10,058,576 B2 “Compositions and Methods Comprising a Defined Microbiome and Methods of Use Thereof.”
Figures

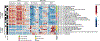
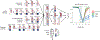


References
-
- Buchfink B, Xie C, and Huson DH (2015). Fast and sensitive protein alignment using DIAMOND. Nat Methods 12(1), 59–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

