Developmental Stage-Specific Changes in Protein Synthesis Differentially Sensitize Hematopoietic Stem Cells and Erythroid Progenitors to Impaired Ribosome Biogenesis
- PMID: 33440178
- PMCID: PMC7815942
- DOI: 10.1016/j.stemcr.2020.11.017
Developmental Stage-Specific Changes in Protein Synthesis Differentially Sensitize Hematopoietic Stem Cells and Erythroid Progenitors to Impaired Ribosome Biogenesis
Abstract
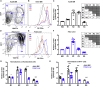
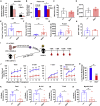
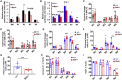
Adult hematopoietic stem cell (HSC) self-renewal requires precise control of protein synthesis, but fetal and adult HSCs have distinct self-renewal mechanisms and lineage outputs. This raises the question of whether protein synthesis rates change with age. Here, we show that protein synthesis rates decline during HSC ontogeny, yet erythroid protein synthesis rates increase. A ribosomal mutation that impairs ribosome biogenesis (Rpl24Bst/+) disrupts both fetal and adult HSC self-renewal. However, the Rpl24Bst/+ mutation selectively impairs fetal erythropoiesis at differentiation stages that exhibit fetal-specific attenuation of protein synthesis. Developmental changes in protein synthesis thus differentially sensitize hematopoietic stem and progenitor cells to impaired ribosome biogenesis.
Keywords: erythroid; erythropoiesis; hematopoiesis; hematopoietic stem cell; progenitor; protein synthesis; proteostasis; ribosome; ribosomopathy; translation.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Akashi K., Traver D., Miyamoto T., Weissman I.L. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. - PubMed
-
- Armistead J., Triggs-Raine B. Diverse diseases from a ubiquitous process: the ribosomopathy paradox. FEBS Lett. 2014;588:1491–1500. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

