Knockdown of TRIM5α or TRIM11 increases lentiviral vector transduction efficiency of human Muller cells
- PMID: 33440192
- PMCID: PMC7946771
- DOI: 10.1016/j.exer.2021.108436
Knockdown of TRIM5α or TRIM11 increases lentiviral vector transduction efficiency of human Muller cells
Abstract
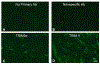
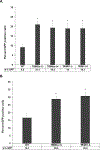
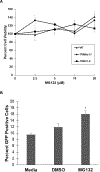
The goal of this study was to determine the expression and distribution of the host restriction factors (RFs) TRIM5α and TRIM11 in non-human primate (NHP) neural retina tissue and the human Muller cell line MIO-M1. In addition, experiments were performed to determine the effect of TRIM5α and TRIM11 knockdown on FIVGFP transduction of MIO-M1 cells with the goal of devising strategies to increase the efficiency of lentiviral (LV) gene delivery. Immunofluorescence (IF) studies indicated that TRIM5α and TRIM11 were localized predominantly in nuclei within the outer nuclear layer (ONL) and inner nuclear layer (INL) of NHP retina tissue. Double label IF indicated that TRIM5α and TRIM11 were localized to some of the retinal Muller cell nuclei. MIO-M1 cells expressed TRIM5α predominantly in the nucleus and TRIM11 primarily in the cytosol. FIVGFP transduction efficiency was significantly increased, at 4 and 7 days post transduction, in TRIM5α and TRIM11 knockdown clones (KD) compared to WT MIO-M1 cells. In addition, pretreatment with the proteasome inhibitor MG132 increased the transduction efficiency of FIVGFP in WT MIO-M1 cells. The nuclear translocation of NF-κB (p65), at 72 h post FIVGFP transduction, was enhanced in TRIM5α and TRIM11 KD clones. The expression of TRIM5α and TRIM11 in macaque neural retina tissue and MIO-M1 cells indicate the presence of these RFs in NHP retina and human Muller cells. Our data indicate that even partial knockdown of TRIM5α or TRIM11, or a short proteasome inhibitor pretreatment, can increase the transduction efficiency of a LV vector.
Keywords: Gene therapy; Muller cells; Proteasome inhibitor; Retina; TRIM11; TRIM5α; Viral vectors.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Figures
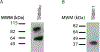

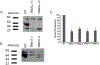

References
-
- Aktas Z, Rao H, Slauson SR, Gabelt BT, Larsen IV, Sheridan RTC, Herrnberger L, Tamm ER, Kaufman PL, Brandt CR: Proteasome inhibition increases the efficiency of lentiviral vector-mediated transduction of trabecular meshwork. Invest. Ophthalmol. Vis. Sci, 59 (2018), pp. 298–310, 10.1167/iovs.17-22074. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

