Ultra-Stable Molecular Sensors by Sub-Micron Referencing and Why They Should Be Interrogated by Optical Diffraction-Part I. The Concept of a Spatial Affinity Lock-in Amplifier
- PMID: 33440783
- PMCID: PMC7827303
- DOI: 10.3390/s21020469
Ultra-Stable Molecular Sensors by Sub-Micron Referencing and Why They Should Be Interrogated by Optical Diffraction-Part I. The Concept of a Spatial Affinity Lock-in Amplifier
Abstract
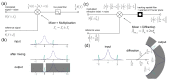
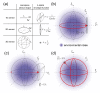
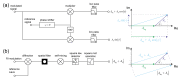
Label-free optical biosensors, such as surface plasmon resonance, are sensitive and well-established for the characterization of molecular interactions. Yet, these sensors require stabilization and constant conditions even with the use of reference channels. In this paper, we use tools from signal processing to show why these sensors are so cross-sensitive and how to overcome their drawbacks. In particular, we conceptualize the spatial affinity lock-in as a universal design principle for sensitive molecular sensors even in the complete absence of stabilization. The spatial affinity lock-in is analogous to the well-established time-domain lock-in. Instead of a time-domain signal, it modulates the binding signal at a high spatial frequency to separate it from the low spatial frequency environmental noise in Fourier space. In addition, direct sampling of the locked-in sensor's response in Fourier space enabled by diffraction has advantages over sampling in real space as done by surface plasmon resonance sensors using the distributed reference principle. This paper and part II hint at the potential of spatially locked-in diffractometric biosensors to surpass state-of-the-art temperature-stabilized refractometric biosensors. Even simple, miniaturized and non-stabilized sensors might achieve the performance of bulky lab instruments. This may enable new applications in label-free analysis of molecular binding and point-of-care diagnostics.
Keywords: chemosensors, biosensors; focal molography; lock-in amplifiers; molecular sensors; noise analysis; noise rejection; optical diffraction.
Conflict of interest statement
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Fraser S., Shih J.Y., Ware M., O’Connor E., Cameron M.J., Schwickart M., Zhao X., Regnstrom K. Current Trends in Ligand Binding Real-Time Measurement Technologies. AAPS J. 2017;19:682–691. - PubMed
-
- Dahlin A.B. Plasmonic Biosensors: An Integrated View of Refractometric Detection. IOS Press; Amsterdam, The Netherlands: 2012.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

