Mitochondrial Reactive Oxygen Species (ROS) Production Alters Sperm Quality
- PMID: 33440836
- PMCID: PMC7827812
- DOI: 10.3390/antiox10010092
Mitochondrial Reactive Oxygen Species (ROS) Production Alters Sperm Quality
Abstract
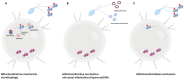
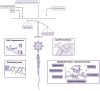
Besides ATP production, mitochondria are key organelles in several cellular functions, such as steroid hormone biosynthesis, calcium homoeostasis, intrinsic apoptotic pathway, and the generation of reactive oxygen species (ROS). Despite the loss of the majority of the cytoplasm occurring during spermiogenesis, mammalian sperm preserves a number of mitochondria that rearrange in a tubular structure at the level of the sperm flagellum midpiece. Although sperm mitochondria are destroyed inside the zygote, the integrity and the functionality of these organelles seem to be critical for fertilization and embryo development. The aim of this review was to discuss the impact of mitochondria-produced ROS at multiple levels in sperm: the genome, proteome, lipidome, epigenome. How diet, aging and environmental pollution may affect sperm quality and offspring health-by exacerbating oxidative stress-will be also described.
Keywords: ROS impact on sperm quality; oxidative stress; sperm physiology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

