Successful gene therapy of Diamond-Blackfan anemia in a mouse model and human CD34+ cord blood hematopoietic stem cells using a clinically applicable lentiviral vector
- PMID: 33440921
- PMCID: PMC8804567
- DOI: 10.3324/haematol.2020.269142
Successful gene therapy of Diamond-Blackfan anemia in a mouse model and human CD34+ cord blood hematopoietic stem cells using a clinically applicable lentiviral vector
Abstract
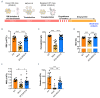
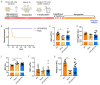
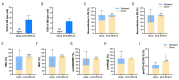
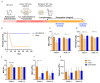
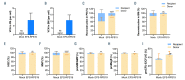
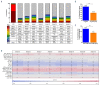
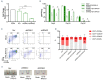
Diamond-Blackfan anemia (DBA) is an inherited bone marrow failure disorder in which pure red blood cell aplasia is associated with physical malformations and a predisposition to cancer. Twentyfive percent of patients with DBA have mutations in a gene encoding ribosomal protein S19 (RPS19). Our previous proof-of-concept studies demonstrated that DBA phenotype could be successfully treated using lentiviral vectors in Rps19-deficient DBA mice. In our present study, we developed a clinically applicable single gene, self-inactivating lentiviral vector, containing the human RPS19 cDNA driven by the human elongation factor 1a short promoter, which can be used for clinical gene therapy development for RPS19-deficient DBA. We examined the efficacy and safety of the vector in a Rps19-deficient DBA mouse model and in human primary RPS19-deficient CD34+ cord blood cells. We observed that transduced Rps19-deficient bone marrow cells could reconstitute mice long-term and rescue the bone marrow failure and severe anemia observed in Rps19-deficient mice, with a low risk of mutagenesis and a highly polyclonal insertion site pattern. More importantly, the vector can also rescue impaired erythroid differentiation in human primary RPS19-deficient CD34+ cord blood hematopoietic stem cells. Collectively, our results demonstrate the efficacy and safety of using a clinically applicable lentiviral vector for the successful treatment of Rps19-deficient DBA in a mouse model and in human primary CD34+ cord blood cells. These findings show that this vector can be used to develop clinical gene therapy for RPS19-deficient DBA patients.
Figures

References
-
- Willig TN, Niemeyer CM, Leblanc T, et al. . Identification of new prognosis factors from the clinical and epidemiologic analysis of a registry of 229 Diamond-Blackfan anemia patients. DBA group of Societe d'Hematologie et d'Immunologie Pediatrique (SHIP), Gesellshaft fur Padiatrische Onkologie und Hamatologie (GPOH), and the European Society for Pediatric Hematology and Immunology (ESPHI). Pediatr Res. 1999;46(5):553-561. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

