Platelet Proteomes, Pathways, and Phenotypes as Informants of Vascular Wellness and Disease
- PMID: 33441027
- PMCID: PMC7980774
- DOI: 10.1161/ATVBAHA.120.314647
Platelet Proteomes, Pathways, and Phenotypes as Informants of Vascular Wellness and Disease
Abstract
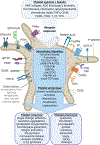
Platelets rapidly undergo responsive transitions in form and function to repair vascular endothelium and mediate hemostasis. In contrast, heterogeneous platelet subpopulations with a range of primed or refractory phenotypes gradually arise in chronic inflammatory and other conditions in a manner that may indicate or support disease. Qualitatively distinguishable platelet phenotypes are increasingly associated with a variety of physiological and pathological circumstances; however, the origins and significance of platelet phenotypic variation remain unclear and conceptually vague. As changes in platelet function in disease exhibit many similarities to platelets following the activation of platelet agonist receptors, the intracellular responses of platelets common to hemostasis and inflammation may provide insights to the molecular basis of platelet phenotype. Here, we review concepts around how protein-level relations-from platelet receptors through intracellular signaling events-may help to define platelet phenotypes in inflammation, immune responses, aging, and other conditions. We further discuss how representing systems-wide platelet proteomics data profiles as circuit-like networks of causally related intracellular events, or, pathway maps, may inform molecular definitions of platelet phenotype. In addition to offering insights into platelets as druggable targets, maps of causally arranged intracellular relations underlying platelet function can also advance precision and interceptive medicine efforts by leveraging platelets as accessible, dynamic, endogenous, circulating biomarkers of vascular wellness and disease. Graphic Abstract: A graphic abstract is available for this article.
Keywords: hemostasis; immunity; inflammation; proteomics; vascular endothelium.
Figures
Similar articles
-
How can we use proteomics to learn more about platelets?Platelets. 2023 Dec;34(1):2217932. doi: 10.1080/09537104.2023.2217932. Platelets. 2023. PMID: 37246523 Free PMC article.
-
Principles of thromboregulation: control of platelet reactivity in vascular disease.Adv Prostaglandin Thromboxane Leukot Res. 1995;23:413-8. Adv Prostaglandin Thromboxane Leukot Res. 1995. PMID: 7732885 Review. No abstract available.
-
Platelet Subtypes in Inflammatory Settings.Front Cardiovasc Med. 2022 Apr 7;9:823549. doi: 10.3389/fcvm.2022.823549. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35463762 Free PMC article. Review.
-
Platelet physiology.Methods Mol Biol. 2013;992:13-30. doi: 10.1007/978-1-62703-339-8_2. Methods Mol Biol. 2013. PMID: 23546702
-
Platelets at work in primary hemostasis.Blood Rev. 2011 Jul;25(4):155-67. doi: 10.1016/j.blre.2011.03.002. Epub 2011 Apr 14. Blood Rev. 2011. PMID: 21496978 Review.
Cited by
-
Analysis of the Healthy Platelet Proteome Identifies a New Form of Domain-Specific O-Fucosylation.Mol Cell Proteomics. 2024 Feb;23(2):100717. doi: 10.1016/j.mcpro.2024.100717. Epub 2024 Jan 16. Mol Cell Proteomics. 2024. PMID: 38237698 Free PMC article.
-
Heterogeneity of platelets and their responses.Res Pract Thromb Haemost. 2024 Mar 1;8(2):102356. doi: 10.1016/j.rpth.2024.102356. eCollection 2024 Feb. Res Pract Thromb Haemost. 2024. PMID: 38666061 Free PMC article.
-
How can we use proteomics to learn more about platelets?Platelets. 2023 Dec;34(1):2217932. doi: 10.1080/09537104.2023.2217932. Platelets. 2023. PMID: 37246523 Free PMC article.
-
Toward platelet transcriptomics in cancer diagnosis, prognosis and therapy.Br J Cancer. 2022 Feb;126(3):316-322. doi: 10.1038/s41416-021-01627-z. Epub 2021 Nov 22. Br J Cancer. 2022. PMID: 34811507 Free PMC article. Review.
-
Salvianolic acid A inhibits the activation and aggregation of platelets in patients with acute coronary syndrome.Medicine (Baltimore). 2025 Jul 18;104(29):e43305. doi: 10.1097/MD.0000000000043305. Medicine (Baltimore). 2025. PMID: 40696601 Free PMC article.
References
-
- Aslan JE. Platelet shape change. In: Gresele P, López J, Kleiman N and Page C, eds. Platelets in Thrombotic and Nonthrombotic Disorders: Springer; 2017.
-
- Steinhubl SR and Moliterno DJ. The role of the platelet in the pathogenesis of atherothrombosis. Am J Cardiovasc Drugs. 2005;5:399–408. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

