Apoptotic Ablation of Platelets Reduces Atherosclerosis in Mice With Diabetes
- PMID: 33441028
- PMCID: PMC7904582
- DOI: 10.1161/ATVBAHA.120.315369
Apoptotic Ablation of Platelets Reduces Atherosclerosis in Mice With Diabetes
Abstract
Objective: People with diabetes are at a significantly higher risk of cardiovascular disease, in part, due to accelerated atherosclerosis. Diabetic subjects have increased number of platelets that are activated, more reactive, and respond suboptimally to antiplatelet therapies. We hypothesized that reducing platelet numbers by inducing their premature apoptotic death would decrease atherosclerosis. Approach and Results: This was achieved by targeting the antiapoptotic protein Bcl-xL (B-cell lymphoma-extra large; which is essential for platelet viability) via distinct genetic and pharmacological approaches. In the former, we transplanted bone marrow from mice carrying the Tyr15 to Cys loss of function allele of Bcl-x (known as Bcl-xPlt20) or wild-type littermate controls into atherosclerotic-prone Ldlr+/- mice made diabetic with streptozotocin and fed a Western diet. Reduced Bcl-xL function in hematopoietic cells significantly decreased platelet numbers, exclusive of other hematologic changes. This led to a significant reduction in atherosclerotic lesion formation in Bcl-xPlt20 bone marrow transplanted Ldlr+/- mice. To assess the potential therapeutic relevance of reducing platelets in atherosclerosis, we next targeted Bcl-xL with a pharmacological strategy. This was achieved by low-dose administration of the BH3 (B-cell lymphoma-2 homology domain 3) mimetic, ABT-737 triweekly, in diabetic Apoe-/- mice for the final 6 weeks of a 12-week study. ABT-737 normalized platelet numbers along with platelet and leukocyte activation to that of nondiabetic controls, significantly reducing atherosclerosis while promoting a more stable plaque phenotype.
Conclusions: These studies suggest that selectively reducing circulating platelets, by targeting Bcl-xL to promote platelet apoptosis, can reduce atherosclerosis and lower cardiovascular disease risk in diabetes. Graphic Abstract: A graphic abstract is available for this article.
Keywords: atherosclerosis; bone marrow; cardiovascular disease; leukocyte; mice.
Figures
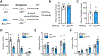



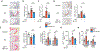
References
-
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. The New England journal of medicine. 1998;339:229–234 - PubMed
-
- Luscher TF, Creager MA, Beckman JA, Cosentino F. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part ii. Circulation. 2003;108:1655–1661 - PubMed
-
- Pasterkamp G Methods of accelerated atherosclerosis in diabetic patients. Heart. 2013;99:743–749 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

