Estrogens and development of the rete testis, efferent ductules, epididymis and vas deferens
- PMID: 33441255
- PMCID: PMC8026493
- DOI: 10.1016/j.diff.2020.11.004
Estrogens and development of the rete testis, efferent ductules, epididymis and vas deferens
Abstract
Estrogen has always been considered the female hormone and testosterone the male hormone. However, estrogen's presence in the testis and deleterious effects of estrogen treatment during development have been known for nearly 90 years, long before estrogen receptors (ESRs) were discovered. Eventually it was learned that testes actually synthesize high levels of estradiol (E2) and sequester high concentrations in the reproductive tract lumen, which seems contradictory to the overwhelming number of studies showing reproductive pathology following exogenous estrogen exposures. For too long, the developmental pathology of estrogen has dominated our thinking, even resulting in the "estrogen hypothesis" as related to the testicular dysgenesis syndrome. However, these early studies and the development of an Esr1 knockout mouse led to a deluge of research into estrogen's potential role in and disruption of development and function of the male reproductive system. What is new is that estrogen action in the male cannot be divorced from that of androgen. This paper presents what is known about components of the estrogen pathway, including its synthesis and target receptors, and the need to achieve a balance between androgen- and estrogen-action in male reproductive tract differentiation and adult functions. The review focuses on what is known regarding development of the male reproductive tract, from the rete testis to the vas deferens, and examines the expression of estrogen receptors and presence of aromatase in the male reproductive system, traces the evidence provided by estrogen-associated knockout and transgenic animal models and discusses the effects of fetal and postnatal exposures to estrogens. Hopefully, there will be enough here to stimulate discussions and new investigations of the androgen:estrogen balance that seems to be essential for development of the male reproductive tract.
Keywords: Development; Differentiation; Efferent ductule; Environmental estrogens; Epididymis; Estrogen; Estrogen receptor; Fetal; Male reproduction; Mesonephric tubule; Mesonephros; Neonatal; Rete cord; Rete testis; Testicular dysgenesis syndrome; Testis; Vas deferens; Wolffian duct.
Copyright © 2020 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Competing interests statements:
Rex Hess has an appointment as Director of Science at Epivara, Inc., a start-up company dedicated to research and development of animal sterilization methods. Epivara provided financial assistance for the literature review but had no influence on the preparation of the manuscript.
Richard Sharpe has no competing interests to declare.
Barry Hinton has no competing interests to declare
Figures





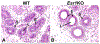

References
-
- Abot A, Fontaine C, Raymond-Letron I, Flouriot G, Adlanmerini M, Buscato M, Otto C, Berges H, Laurell H, Gourdy P, Lenfant F, Arnal JF, 2013. The AF-1 activation function of estrogen receptor alpha is necessary and sufficient for uterine epithelial cell proliferation in vivo. Endocrinology 154, 2222–2233. - PubMed
-
- Aceitero J, Llanero M, Parrado R, Pena E, Lopez-Beltran A, 1998. Neonatal exposure of male rats to estradiol benzoate causes rete testis dilation and backflow impairment of spermatogenesis. Anat Rec 252, 17–33. - PubMed
-
- Ahlbory-Dieker DL, Stride BD, Leder G, Schkoldow J, Trolenberg S, Seidel H, Otto C, Sommer A, Parker MG, Schutz G, Wintermantel TM, 2009. DNA binding by estrogen receptor-alpha is essential for the transcriptional response to estrogen in the liver and the uterus. Mol Endocrinol 23, 1544–1555. - PMC - PubMed
-
- Amann RP, Ganjam VK, 1976. Steroid production by the bovine testis and steroid transfer across the pampiniform plexus. Biol Reprod 15, 695–703. - PubMed
-
- Amano A, Kondo Y, Noda Y, Ohta M, Kawanishi N, Machida S, Mitsuhashi K, Senmaru T, Fukui M, Takaoka O, Mori T, Kitawaki J, Ono M, Saibara T, Obayashi H, Ishigami A, 2017. Abnormal lipid/lipoprotein metabolism and high plasma testosterone levels in male but not female aromatase-knockout mice. Archives of biochemistry and biophysics 622, 47–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

