Cross-Reactive anti-Nucleocapsid Protein Immunity against Crimean-Congo Hemorrhagic Fever Virus and Hazara Virus in Multiple Species
- PMID: 33441341
- PMCID: PMC8092682
- DOI: 10.1128/JVI.02156-20
Cross-Reactive anti-Nucleocapsid Protein Immunity against Crimean-Congo Hemorrhagic Fever Virus and Hazara Virus in Multiple Species
Abstract
The World Health Organization estimates that there may be three billion people at risk of infection by Crimean-Congo Hemorrhagic Fever Virus (CCHFV), a highly lethal, emerging orthonairovirus carried by ticks. On the other hand, the closely related Hazara virus (HAZV), a member of the same serogroup, has not been reported as a pathogen for humans. Given the structural and phylogenetic similarities between these two viruses, we evaluated the immunological similarities of the nucleocapsid protein (NP) of these two viruses in multiple species. Strong antigenic similarities were demonstrated in anti-NP humoral immune responses against HAZV and CCHFV in multiple species using convalescent human CCHF sera, rabbit and mouse polyclonal antiserum raised against CCHFV, and mouse polyclonal antiserum against CCHFV-NP in enzyme immunoassays. We also report a convincing cross-reactivity between NPs in Western blots using HAZV-infected cell lysate as antigen and inactivated CCHFV and CCHFV-NP-immunized mice sera. These results suggest that NPs of HAZV and CCHFV share significant similarities in humoral responses across species and underline the potential utility of HAZV as a surrogate model for CCHFV.IMPORTANCE CCHFV and HAZV, members of the Nairoviridae family, are transmitted to mammals by tick bites. CCHFV is considered to be a severe threat to public health and causes hemorrhagic diseases with a high mortality rate, and there are neither preventative nor therapeutic medications against CCHFV disease. HAZV, on the other hand, is not a pathogen to humans and can be studied under BSL-2 conditions. The antigenic relationship between these viruses is of interest for vaccines and for preventative investigations. Here, we demonstrate cross-reactivity in anti-NP humoral immune response between NPs of HAZV and CCHFV in multiple species. These results underline the utility of HAZV as a surrogate model to study CCHFV infection.
Copyright © 2021 American Society for Microbiology.
Figures
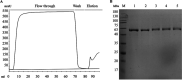

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

