Experimental Reptarenavirus Infection of Boa constrictor and Python regius
- PMID: 33441344
- PMCID: PMC8092697
- DOI: 10.1128/JVI.01968-20
Experimental Reptarenavirus Infection of Boa constrictor and Python regius
Abstract
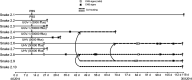
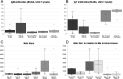
Boid inclusion body disease (BIBD) causes losses in captive snake populations globally. BIBD is associated with the formation of cytoplasmic inclusion bodies (IBs), which mainly comprise reptarenavirus nucleoprotein (NP). In 2017, BIBD was reproduced by cardiac injection of boas and pythons with reptarenaviruses, thus demonstrating a causative link between reptarenavirus infection and the disease. Here, we report experimental infections of Python regius (n = 16) and Boa constrictor (n = 16) with three reptarenavirus isolates. First, we used pythons (n = 8) to test two virus delivery routes: intraperitoneal injection and tracheal instillation. Viral RNAs but no IBs were detected in brains and lungs at 2 weeks postinoculation. Next, we inoculated pythons (n = 8) via the trachea. During the 4 months following infection, snakes showed transient central nervous system (CNS) signs but lacked detectable IBs at the time of euthanasia. One of the snakes developed severe CNS signs; we succeeded in reisolating the virus from the brain of this individual and could demonstrate viral antigen in neurons. In a third attempt, we tested cohousing, vaccination, and sequential infection with multiple reptarenavirus isolates on boas (n = 16). At 10 months postinoculation, all but one snake tested positive for viral RNA in lung, brain, and/or blood, but none exhibited the characteristic IBs. Three of the four vaccinated snakes seemed to sustain challenge with the same reptarenavirus; however, neither of the two snakes rechallenged with different reptarenaviruses remained uninfected. Comparison of the antibody responses in experimentally versus naturally reptarenavirus-infected animals indicated differences in the responses.IMPORTANCE In the present study, we experimentally infected pythons and boas with reptarenavirus via either intraperitoneal injection or tracheal instillation. The aims were to experimentally induce boid inclusion body disease (BIBD) and to develop an animal model for studying disease transmission and pathogenesis. Both virus delivery routes resulted in infection, and infection via the trachea could reflect the natural route of infection. In the experimentally infected snakes, we did not find evidence of inclusion body (IB) formation, characteristic of BIBD, in pythons or boas. Most of the boas (11/12) remained reptarenavirus infected after 10 months, which suggests that they developed a persistent infection that could eventually have led to BIBD. We demonstrated that vaccination using recombinant protein or an inactivated virus preparation prevented infection by a homologous virus in three of four snakes. Comparison of the antibody responses of experimentally and naturally reptarenavirus-infected snakes revealed differences that merit further studies.
Copyright © 2021 American Society for Microbiology.
Figures



Similar articles
-
Boid Inclusion Body Disease Is Also a Disease of Wild Boa Constrictors.Microbiol Spectr. 2022 Oct 26;10(5):e0170522. doi: 10.1128/spectrum.01705-22. Epub 2022 Sep 12. Microbiol Spectr. 2022. PMID: 36094085 Free PMC article.
-
Identification of Reptarenaviruses, Hartmaniviruses, and a Novel Chuvirus in Captive Native Brazilian Boa Constrictors with Boid Inclusion Body Disease.J Virol. 2020 May 18;94(11):e00001-20. doi: 10.1128/JVI.00001-20. Print 2020 May 18. J Virol. 2020. PMID: 32238580 Free PMC article.
-
Reptarenavirus S Segment RNA Levels Correlate with the Presence of Inclusion Bodies and the Number of L Segments in Snakes with Reptarenavirus Infection-Lessons Learned from a Large Breeding Colony.Microbiol Spectr. 2023 Jun 15;11(3):e0506522. doi: 10.1128/spectrum.05065-22. Epub 2023 May 22. Microbiol Spectr. 2023. PMID: 37212675 Free PMC article.
-
Mammarenavirus Genetic Diversity and Its Biological Implications.Curr Top Microbiol Immunol. 2023;439:265-303. doi: 10.1007/978-3-031-15640-3_8. Curr Top Microbiol Immunol. 2023. PMID: 36592249 Review.
-
Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus.Avian Pathol. 2003 Dec;32(6):567-82. doi: 10.1080/03079450310001621198. Avian Pathol. 2003. PMID: 14676007 Free PMC article. Review.
Cited by
-
A Multiplex RT-PCR Method for the Detection of Reptarenavirus Infection.Viruses. 2023 Nov 25;15(12):2313. doi: 10.3390/v15122313. Viruses. 2023. PMID: 38140554 Free PMC article.
-
Molecular characterization of a reptarenavirus detected in a Colombian Red-Tailed Boa (Boa constrictor imperator).Virol J. 2023 Nov 15;20(1):265. doi: 10.1186/s12985-023-02237-2. Virol J. 2023. PMID: 37968659 Free PMC article.
-
Persistent Reptarenavirus and Hartmanivirus Infection in Cultured Boid Cells.Microbiol Spectr. 2022 Aug 31;10(4):e0158522. doi: 10.1128/spectrum.01585-22. Epub 2022 Jul 7. Microbiol Spectr. 2022. PMID: 35862992 Free PMC article.
-
Multifocal cutaneous neoplastic vascular proliferations in a rainbow boa (Epicrates cenchria) collection with boid inclusion body disease.PLoS One. 2024 Nov 6;19(11):e0311015. doi: 10.1371/journal.pone.0311015. eCollection 2024. PLoS One. 2024. PMID: 39504334 Free PMC article.
-
Temperature affects reptarenavirus growth in a permissive host-derived in vitro model.J Gen Virol. 2025 Apr;106(4):002100. doi: 10.1099/jgv.0.002100. J Gen Virol. 2025. PMID: 40299760 Free PMC article.
References
-
- Chang L, Jacobson ER. 2010. Inclusion body disease, a worldwide infectious disease of boid snakes: a review. J Exot Pet Med 19:216–225. doi:10.1053/j.jepm.2010.07.014. - DOI
-
- Schumacher J, Jacobson ER, Homer BL, Gaskin JM. 1994. Inclusion body disease in boid snakes. J Zoo Wildl Med 25:511–524.
-
- Wozniak E, McBride J, DeNardo D, Tarara R, Wong V, Osburn B. 2000. Isolation and characterization of an antigenically distinct 68-kd protein from nonviral intracytoplasmic inclusions in boa constrictors chronically infected with the inclusion body disease virus (IBDV: Retroviridae). Vet Pathol 37:449–459. doi:10.1354/vp.37-5-449. - DOI - PubMed
-
- Chang LW, Fu A, Wozniak E, Chow M, Duke DG, Green L, Kelley K, Hernandez JA, Jacobson ER. 2013. Immunohistochemical detection of a unique protein within cells of snakes having inclusion body disease, a world-wide disease seen in members of the families Boidae and Pythonidae. PLoS One 8:e82916. doi:10.1371/journal.pone.0082916. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

