Non-steroidal anti-inflammatory drugs dampen the cytokine and antibody response to SARS-CoV-2 infection
- PMID: 33441348
- PMCID: PMC8092681
- DOI: 10.1128/JVI.00014-21
Non-steroidal anti-inflammatory drugs dampen the cytokine and antibody response to SARS-CoV-2 infection
Abstract
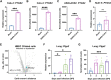
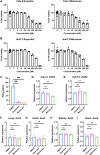
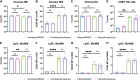
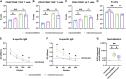
Identifying drugs that regulate severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and its symptoms has been a pressing area of investigation during the coronavirus disease 2019 (COVID-19) pandemic. Nonsteroidal anti-inflammatory drugs (NSAIDs), which are frequently used for the relief of pain and inflammation, could modulate both SARS-CoV-2 infection and the host response to the virus. NSAIDs inhibit the enzymes cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2), which mediate the production of prostaglandins (PGs). As PGs play diverse biological roles in homeostasis and inflammatory responses, inhibiting PG production with NSAIDs could affect COVID-19 pathogenesis in multiple ways, including: (1) altering susceptibility to infection by modifying expression of angiotensin-converting enzyme 2 (ACE2), the cell entry receptor for SARS-CoV-2; (2) regulating replication of SARS-CoV-2 in host cells; and (3) modulating the immune response to SARS-CoV-2. Here, we investigate these potential roles. We demonstrate that SARS-CoV-2 infection upregulates COX-2 in diverse human cell culture and mouse systems. However, suppression of COX-2 by two commonly used NSAIDs, ibuprofen and meloxicam, had no effect on ACE2 expression, viral entry, or viral replication. In contrast, in a mouse model of SARS-CoV-2 infection, NSAID treatment reduced production of pro-inflammatory cytokines and impaired the humoral immune response to SARS-CoV-2 as demonstrated by reduced neutralizing antibody titers. Our findings indicate that NSAID treatment may influence COVID-19 outcomes by dampening the inflammatory response and production of protective antibodies rather than modifying susceptibility to infection or viral replication.ImportancePublic health officials have raised concerns about the use of nonsteroidal anti-inflammatory drugs (NSAIDs) for treating symptoms of coronavirus disease 2019 (COVID-19). NSAIDs inhibit the enzymes cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2), which are critical for the generation of prostaglandins - lipid molecules with diverse roles in homeostasis and inflammation. Inhibition of prostaglandin production by NSAIDs could therefore have multiple effects on COVID-19 pathogenesis. Here, we demonstrate that NSAID treatment reduced both the antibody and pro-inflammatory cytokine response to SARS-CoV-2 infection. The ability of NSAIDs to modulate the immune response to SARS-CoV-2 infection has important implications for COVID-19 pathogenesis in patients. Whether this occurs in humans and whether it is beneficial or detrimental to the host remains an important area of future investigation. This also raises the possibility that NSAIDs may alter the immune response to SARS-CoV-2 vaccination.
Copyright © 2021, American Society for Microbiology.
Figures



References
-
- Day M. 2020. Covid-19: ibuprofen should not be used for managing symptoms, say doctors and scientists. BMJ 368:m1086. - PubMed
-
- Powis S. 2020. Novel coronavirus: anti-inflammatory medications. Medicines and Healthcare Products Regulatory Agency, London, United Kingdom.
-
- Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, Si H-R, Zhu Y, Li B, Huang C-L, Chen H-D, Chen J, Luo Y, Guo H, Jiang R-D, Liu M-Q, Chen Y, Shen X-R, Wang X, Zheng X-S, Zhao K, Chen Q-J, Deng F, Liu L-L, Yan B, Zhan F-X, Wang Y-Y, Xiao G-F, Shi Z-L. 2020. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579:270–273. doi: 10.1038/s41586-020-2012-7. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

