Enhancement of Opioid Antinociception by Nicotinic Ligands
- PMID: 33441370
- PMCID: PMC7985615
- DOI: 10.1124/jpet.120.000423
Enhancement of Opioid Antinociception by Nicotinic Ligands
Abstract
Nicotine has previously been shown to augment the antinociceptive effects of μ-opioid agonists in squirrel monkeys without producing a concomitant increase in behavioral disruption. The present studies were conducted to extend these findings by determining the ability of the nicotinic acetylcholine receptor (nAChR) agonist epibatidine and partial α4β2 nAChR agonist varenicline to selectively augment the antinociceptive effects of the μ-opioid receptor (MOR) full agonist fentanyl, the MOR partial agonist nalbuphine, and the κ-opioid receptor (KOR) agonist U69,593 in male squirrel monkeys. Results indicate that both nAChR ligands selectively increased the antinociceptive effects of nalbuphine and that epibatidine increased the antinociceptive effects of U69,593 without altering effects on operant behavior. However, neither epibatidine nor varenicline enhanced the antinociceptive effects of fentanyl, perhaps due to its high efficacy. The enhancement of nalbuphine's antinociceptive effects by epibatidine, but not varenicline, could be antagonized by either mecamylamine or dihydro-β-erythroidine, consistent with α4β2 mediation of epibatidine's effects but suggesting the involvement of non-nAChR mechanisms in the effects of varenicline. The present results support previous findings showing that an nAChR agonist can serve as an adjuvant for MOR antinociception and, based on results with U69,593, further indicate that the adjuvant effects of nAChR drugs may also apply to antinociception produced by KOR. Our findings support the further evaluation of nAChR agonists as adjuvants of opioid pharmacotherapy for pain management and point out the need for further investigation into the mechanisms by which they produce opioid-adjuvant effects. SIGNIFICANCE STATEMENT: Nicotine has been shown to augment the antinociceptive effects of μ-opioid receptor analgesics without exacerbating their effects on operant performance. The present study demonstrates that the nicotinic acetylcholine receptor (nAChR) agonist epibatidine and partial α4β2 nAChR agonist varenicline can also augment the antinociceptive effects of nalbuphine, as well as those of a κ-opioid receptor agonist, without concomitantly exacerbating their behaviorally disruptive effects. These findings support the view that nAChR agonists and partial agonists may have potential as adjuvant therapies for opioid-based analgesics.
Copyright © 2021 by The American Society for Pharmacology and Experimental Therapeutics.
Conflict of interest statement
No author has an actual or perceived conflict of interest with the contents of this article.
Figures



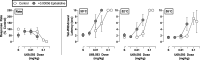
Similar articles
-
The contribution of α4β2 and non-α4β2 nicotinic acetylcholine receptors to the discriminative stimulus effects of nicotine and varenicline in mice.Psychopharmacology (Berl). 2017 Mar;234(5):781-792. doi: 10.1007/s00213-016-4514-4. Epub 2016 Dec 27. Psychopharmacology (Berl). 2017. PMID: 28028600 Free PMC article.
-
Enhancement of Opioid Antinociception by Nicotine.J Pharmacol Exp Ther. 2019 Dec;371(3):624-632. doi: 10.1124/jpet.119.261438. Epub 2019 Sep 16. J Pharmacol Exp Ther. 2019. PMID: 31527281 Free PMC article.
-
Involvement of Nicotinic Receptor Subtypes in the Behavioral Effects of Nicotinic Drugs in Squirrel Monkeys.J Pharmacol Exp Ther. 2018 Aug;366(2):397-409. doi: 10.1124/jpet.118.248070. Epub 2018 May 21. J Pharmacol Exp Ther. 2018. PMID: 29784663 Free PMC article.
-
Therapeutic potential of neuronal nicotinic acetylcholine receptor agonists as novel analgesics.Biochem Pharmacol. 1999 Sep 15;58(6):917-23. doi: 10.1016/s0006-2952(99)00122-7. Biochem Pharmacol. 1999. PMID: 10509744 Review.
-
The contribution of agonist and antagonist activities of α4β2* nAChR ligands to smoking cessation efficacy: a quantitative analysis of literature data.Psychopharmacology (Berl). 2018 Sep;235(9):2479-2505. doi: 10.1007/s00213-018-4921-9. Epub 2018 Jul 7. Psychopharmacology (Berl). 2018. PMID: 29980822 Review.
References
-
- Blaudszun G, Lysakowski C, Elia N, Tramèr MR (2012) Effect of perioperative systemic α2 agonists on postoperative morphine consumption and pain intensity: systematic review and meta-analysis of randomized controlled trials. Anesthesiology 116:1312–1322. - PubMed
-
- Butelman ER, Ball JW, Kreek MJ (2004) Peripheral selectivity and apparent efficacy of dynorphins: comparison to non-peptidic kappa-opioid agonists in rhesus monkeys. Psychoneuroendocrinology 29:307–326. - PubMed
-
- Clark DJ, Schumacher MA (2017) America’s opioid epidemic: supply and demand considerations. Anesth Analg 125:1667–1674. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

