Rationale for azithromycin in COVID-19: an overview of existing evidence
- PMID: 33441373
- PMCID: PMC7811960
- DOI: 10.1136/bmjresp-2020-000806
Rationale for azithromycin in COVID-19: an overview of existing evidence
Abstract
Azithromycin has rapidly been adopted as a repurposed drug for the treatment of COVID-19, despite the lack of high-quality evidence. In this review, we critically appraise the current pharmacological, preclinical and clinical data of azithromycin for treating COVID-19. Interest in azithromycin has been fuelled by favourable treatment outcomes in other viral pneumonias, a documented antiviral effect on SARS-CoV-2 in vitro and uncontrolled case series early in the pandemic. Its antiviral effects presumably result from interfering with receptor mediated binding, viral lysosomal escape, intracellular cell-signalling pathways and enhancing type I and III interferon expression. Its immunomodulatory effects may mitigate excessive inflammation and benefit tissue repair. Currently, in vivo reports on azithromycin in COVID-19 are conflicting and do not endorse its widespread use outside of clinical trials. They are, however, mostly retrospective and therefore inherently biased. The effect size of azithromycin may depend on when it is started. Also, extended follow-up is needed to assess benefits in the recovery phase. Safety data warrant monitoring of drug-drug interactions and subsequent cardiac adverse events, especially with hydroxychloroquine. More prospective data of large randomised controlled studies are expected and much-needed. Uniform reporting of results should be strongly encouraged to facilitate data pooling with the many ongoing initiatives.
Keywords: COVID-19; respiratory infection; viral infection.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: IG has nothing to disclose. WJ reports grants from Research Fund Flanders (FWO), grants and personal fees from Astra Zeneca, grants and personal fees from Chiesi, and is cofounder of ArtiQ, outside the submitted work. PV has nothing to disclose. RV reports grants from Research Foundation Flanders, outside the submitted work. There was no specific funding for this manuscript. The manuscript, the abstract or the figures have never been published or presented.
Figures
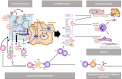

 Azithromycin stimulatory and inhibitory immunomodulatory effects. Ang II, angiotensin I; CCL5, C-C motif chemokine ligand 5 (=RANTES); CTL, cytotoxic T-cell; CXCL, C-X-C motif chemokine ligand; DAMP, danger associated molecular pattern, GMCSF, granulocyte macrophage colony stimulating factor; IFN, interferon, IL, interleukin; IRF, interferon inducible factors; NET, neutrophil extracellular traps; NF-KB, nuclear factor kappa beta; NK, natural killer cell; NLRP3, nod-like receptor pyrin domain containing 3; P2RX, purinergic receptor P2X; PAMP, pathogen associated molecular pattern; PDGF, platelet-derived growth factor; RIG, retinoic acid inducible gene 1; Th, T helper cell; TLR, toll like receptor; TNF, tumour necrosis factor.
Azithromycin stimulatory and inhibitory immunomodulatory effects. Ang II, angiotensin I; CCL5, C-C motif chemokine ligand 5 (=RANTES); CTL, cytotoxic T-cell; CXCL, C-X-C motif chemokine ligand; DAMP, danger associated molecular pattern, GMCSF, granulocyte macrophage colony stimulating factor; IFN, interferon, IL, interleukin; IRF, interferon inducible factors; NET, neutrophil extracellular traps; NF-KB, nuclear factor kappa beta; NK, natural killer cell; NLRP3, nod-like receptor pyrin domain containing 3; P2RX, purinergic receptor P2X; PAMP, pathogen associated molecular pattern; PDGF, platelet-derived growth factor; RIG, retinoic acid inducible gene 1; Th, T helper cell; TLR, toll like receptor; TNF, tumour necrosis factor.References
-
- WHO Coronavirus disease 2019 (COVID-19) situation report–107. 2020. Available: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situatio...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
