Therapy of lymphoma by immune checkpoint inhibitors: the role of T cells, NK cells and cytokine-induced tumor senescence
- PMID: 33441389
- PMCID: PMC7812096
- DOI: 10.1136/jitc-2020-001660
Therapy of lymphoma by immune checkpoint inhibitors: the role of T cells, NK cells and cytokine-induced tumor senescence
Abstract
Background: Although antibodies blocking immune checkpoints have already been approved for clinical cancer treatment, the mechanisms involved are not yet completely elucidated. Here we used a λ-MYC transgenic model of endogenously growing B-cell lymphoma to analyze the requirements for effective therapy with immune checkpoint inhibitors.
Methods: Growth of spontaneous lymphoma was monitored in mice that received antibodies targeting programmed cell death protein 1 and cytotoxic T lymphocyte-associated protein-4, and the role of different immune cell compartments and cytokines was studied by in vivo depletion experiments. Activation of T and natural killer cells and the induction of tumor senescence were analyzed by flow cytometry.
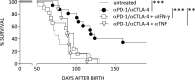
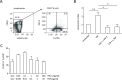
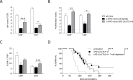
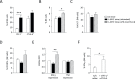
Results: On immune checkpoint blockade, visible lymphomas developed at later time points than in untreated controls, indicating an enhanced tumor control. Importantly, 20% to 30% of mice were even long-term protected and did never develop clinical signs of tumor growth. The therapeutic effect was dependent on cytokine-induced senescence in malignant B cells. The proinflammatory cytokines interferon-γ (IFN-γ) and tumor necrosis factor (TNF) were necessary for the survival benefit as well as for senescence induction in the λ-MYC model. Antibody therapy improved T-cell functions such as cytokine production, and long-time survivors were only observed in the presence of T cells. Yet, NK cells also had a pronounced effect on therapy-induced delay of tumor growth. Antibody treatment enhanced numbers, proliferation and IFN-γ expression of NK cells in developing tumors. The therapeutic effect was fully abrogated only after depletion of both, T cells and NK cells, or after ablation of either IFN-γ or TNF.
Conclusions: Tumor cell senescence may explain why patients responding to immune checkpoint blockade frequently show stable growth arrest of tumors rather than complete tumor regression. In the lymphoma model studied, successful therapy required both, tumor-directed T-cell responses and NK cells, which control, at least partly, tumor development through cytokine-induced tumor senescence.
Keywords: CTLA-4 antigen; hematologic neoplasms; immunotherapy; programmed cell death 1 receptor; tumor microenvironment.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
