Correlation of SARS-CoV-2 Nucleocapsid Antigen and RNA Concentrations in Nasopharyngeal Samples from Children and Adults Using an Ultrasensitive and Quantitative Antigen Assay
- PMID: 33441395
- PMCID: PMC8092747
- DOI: 10.1128/JCM.03077-20
Correlation of SARS-CoV-2 Nucleocapsid Antigen and RNA Concentrations in Nasopharyngeal Samples from Children and Adults Using an Ultrasensitive and Quantitative Antigen Assay
Abstract
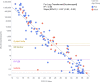
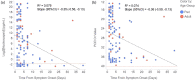
Diagnosis of COVID-19 by PCR offers high sensitivity, but the utility of detecting samples with high cycle threshold (CT ) values remains controversial. Currently available rapid diagnostic tests (RDTs) for SARS-CoV-2 nucleocapsid antigens (Ag) have sensitivity well below PCR. The correlation of Ag and RNA quantities in clinical nasopharyngeal (NP) samples is unknown. An ultrasensitive, quantitative electrochemiluminescence immunoassay for SARS-CoV-2 nucleocapsid (the MSD S-PLEX SARS-CoV-2 N assay) was used to measure Ag in clinical NP samples from adults and children previously tested by PCR. The S-PLEX Ag assay had a limit of detection (LOD) of 0.16 pg/ml and a cutoff of 0.32 pg/ml. Ag concentrations measured in clinical NP samples (collected in 3.0 ml of media) ranged from less than 160 fg/ml to 2.7 μg/ml. Log-transformed Ag concentrations correlated tightly with CT values. In 35 adult and 101 pediatric PCR-positive samples, the sensitivities were 91% (95% confidence interval, 77 to 98%) and 79% (70 to 87%), respectively. In samples with a CT of ≤35, the sensitivities were 100% (88 to 100%) and 96% (88 to 99%), respectively. In 50 adult and 40 pediatric PCR-negative specimens, the specificities were 100% (93 to 100%) and 98% (87 to 100%), respectively. Nucleocapsid concentrations in clinical NP samples span 8 orders of magnitude and correlate closely with RNA concentrations (CT values). The S-PLEX Ag assay showed 96 to 100% sensitivity in samples from children and adults with CT values of ≤35, and a specificity of 98 to 100%. These results clarify Ag concentration distributions in clinical samples, providing insight into the performance of Ag RDTs and offering a new approach to diagnosis of COVID-19.
Keywords: COVID-19; SARS-CoV-2; antigen; diagnostic; nucleoprotein.
Copyright © 2021 American Society for Microbiology.
Figures

References
-
- Center for Systems Science and Engineering. 2020. COVID-19 dashboard by the Center for Systems Science and Engineering (CCSE) at Johns Hopkins University (JHU). https://coronavirus.jhu.edu/map.html. Accessed 31 October 2020.
-
- Centers for Disease Control. 2020. COVID data tracker. Centers for Disease Control and Prevention, Atlanta, GA. https://covid.cdc.gov/covid-data-tracker/#testing_testsperformed. Accessed 31 October 2020.
-
- Krüger LJ, Gaeddert M, Köppel L, Brümmer LE, Gottschalk C, Miranda IB, Schnitzler P, Kräusslich HG, Lindner AK, Nikolai O, Mockenhaupt FP, Seybold J, Corman VM, Drosten C, Pollock NR, Cubas-Atienzar AI, Kontogianni K, Collins A, Wright AH, Knorr B, Welker A, de Vos M, Sacks JA, Adams ER, Denkinger CM. 2020. Evaluation of the accuracy, ease of use and limit of detection of novel, rapid, antigen-detecting point-of-care diagnostics for SARS-CoV-2. medRxiv doi: 10.1101/2020.10.01.20203836:2020.10.01.20203836. - DOI
-
- Lindner AK, Nikolai O, Kausch F, Wintel M, Hommes F, Gertler M, Krüger L, Gaeddert M, Tobian F, Lainati F, Köppel L, Seybold J, Corman VM, Drosten C, Hofmann J, Sacks J, Mockenhaupt F, Denkinger CM. 2020. Head-to-head comparison of SARS-CoV-2 antigen-detecting rapid test with self-collected anterior nasal swab versus professional-collected nasopharyngeal swab. medRxiv doi: 10.1101/2020.10.26.20219600:2020.10.26.20219600. - DOI - PMC - PubMed
-
- FDA. 2020. Package insert for the BD VeritorTM system for rapid detection of SARS-CoV-2. Food and Drug Administration, Washington, DC. https://www.fda.gov/media/139755/download. Accessed 2 November 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

