One-year intensive lifestyle intervention and improvements in health-related quality of life and mental health in persons with type 2 diabetes: a secondary analysis of the U-TURN randomized controlled trial
- PMID: 33441418
- PMCID: PMC7812095
- DOI: 10.1136/bmjdrc-2020-001840
One-year intensive lifestyle intervention and improvements in health-related quality of life and mental health in persons with type 2 diabetes: a secondary analysis of the U-TURN randomized controlled trial
Abstract
Introduction: The effects of lifestyle interventions in persons with type 2 diabetes (T2D) on health-related quality of life (HRQoL) and subjective well-being are ambiguous, and no studies have explored the effect of exercise interventions that meet or exceed current recommended exercise levels. We investigated whether a 1-year intensive lifestyle intervention is superior in improving HRQoL compared with standard care in T2D persons.
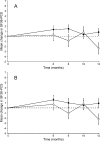
Research design and methods: We performed secondary analyses of a previously conducted randomized controlled trial (April 2015 to August 2016). Persons with non-insulin-dependent T2D (duration ≤10 years) were randomized to 1-year supervised exercise and individualized dietary counseling (ie, 'U-TURN'), or standard care. The primary HRQoL outcome was change in the 36-item Short Form Health Survey (SF-36) physical component score (PCS) from baseline to 12 months of follow-up, and a key secondary outcome was changes in the SF-36 mental component score (MCS).
Results: We included 98 participants (U-TURN group=64, standard care group=34) with a mean age of 54.6 years (SD 8.9). Between-group analyses at 12-month follow-up showed SF-36 PCS change of 0.8 (95% CI -0.7 to 2.3) in the U-TURN group and deterioration of 2.4 (95% CI -4.6 to -0.1) in the standard care group (difference of 3.2, 95% CI 0.5 to 5.9, p=0.02) while no changes were detected in SF-36 MCS. At 12 months, 19 participants (30%) in the U-TURN group and 6 participants (18%) in the standard care group achieved clinically significant improvement in SF-36 PCS score (adjusted risk ratio 2.6, 95% CI 1.0 to 4.5 corresponding to number needed to treat of 4, 95% CI 1.6 to infinite).
Conclusion: In persons with T2D diagnosed for less than 10 years, intensive lifestyle intervention improved the physical component of HRQoL, but not the mental component of HRQoL after 1 year, compared with standard care.
Trial registration number: NCT02417012.
Keywords: diabetes mellitus; exercise; life style; quality of life; type 2.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: AV was appointed vice-president for AstraZeneca’s Translational Research and Early Clinical Development during the completion of the study, but remained in the scientific steering committee of this study. RC and SMN’s employer, the Parker Institute, Bispebjerg and Frederiksberg Hospital, is supported by core grant OCAY-13-309 from the Oak Foundation. RC reports receiving personal fees from Abbott, AbbVie, Amgen, Axellus, Bayer HealthCare Pharmaceuticals, Biogen Idec, Bristol-Myers Squibb, Cambridge Weight Plan, Celgene, Eli Lilly, Hospira, Ipsen, Janssen, Laboratoires Expanscience, and Merck Sharp; personal fees from employment from Research Unit for Musculoskeletal Function and Physiotherapy, Institute of Sports Science and Clinical Biomechanics, and the University of Southern Denmark; grants pending and grant funding from Axellus, AbbVie, Cambridge Weight Plan, Janssen, and Merck Sharp; and being involved in many healthcare initiatives and research that could benefit from wide uptake of this publication, including Cochrane, Outcome Measures in Rheumatology, International Dermatology Outcome Measures, RADS, and the Grading of Recommendations Assessment, Development and Evaluation Working Group. MRL received personal speaker fees from Novo Nordisk.
Figures
Similar articles
-
The Effects of a Lifestyle Intervention Supported by the InterWalk Smartphone App on Increasing Physical Activity Among Persons With Type 2 Diabetes: Parallel-Group, Randomized Trial.JMIR Mhealth Uhealth. 2022 Sep 28;10(9):e30602. doi: 10.2196/30602. JMIR Mhealth Uhealth. 2022. PMID: 36170002 Free PMC article. Clinical Trial.
-
Impact of lifestyle intervention and metformin on health-related quality of life: the diabetes prevention program randomized trial.J Gen Intern Med. 2012 Dec;27(12):1594-601. doi: 10.1007/s11606-012-2122-5. Epub 2012 Jun 13. J Gen Intern Med. 2012. PMID: 22692637 Free PMC article. Clinical Trial.
-
Effect of an Intensive Lifestyle Intervention on Glycemic Control in Patients With Type 2 Diabetes: A Randomized Clinical Trial.JAMA. 2017 Aug 15;318(7):637-646. doi: 10.1001/jama.2017.10169. JAMA. 2017. PMID: 28810024 Free PMC article. Clinical Trial.
-
Head-to-head comparison of intensive lifestyle intervention (U-TURN) versus conventional multifactorial care in patients with type 2 diabetes: protocol and rationale for an assessor-blinded, parallel group and randomised trial.BMJ Open. 2015 Dec 9;5(12):e009764. doi: 10.1136/bmjopen-2015-009764. BMJ Open. 2015. PMID: 26656025 Free PMC article. Clinical Trial.
-
Lifestyle Intervention for Diabetes Remission: A Paradigm Shift inDiabetes Care and Management.Curr Diabetes Rev. 2024;20(9):e291123224009. doi: 10.2174/0115733998272795231109034141. Curr Diabetes Rev. 2024. PMID: 38093443 Review.
Cited by
-
Mediterranean Diet and Physical Activity Nudges versus Usual Care in Women with Rheumatoid Arthritis: Results from the MADEIRA Randomized Controlled Trial.Nutrients. 2023 Jan 28;15(3):676. doi: 10.3390/nu15030676. Nutrients. 2023. PMID: 36771382 Free PMC article. Clinical Trial.
-
Diabetic Foot Ulcer Beyond Wound Closure: Clinical Practice Guideline.Phys Ther. 2025 Jan 8;105(1):pzae171. doi: 10.1093/ptj/pzae171. Phys Ther. 2025. PMID: 39574416 Free PMC article.
-
Physical activity, and improvement in health-related quality of life among Australian middle-aged and older adults living with type 2 diabetes mellitus.Qual Life Res. 2025 Apr;34(4):1027-1043. doi: 10.1007/s11136-024-03865-z. Epub 2024 Dec 5. Qual Life Res. 2025. PMID: 39636511
-
Feasibility of a 12 weeks supervised exercise training intervention among people with Maturity Onset Diabetes of the Young (MODY) or type 2 diabetes in Greenland.Int J Circumpolar Health. 2024 Dec;83(1):2403794. doi: 10.1080/22423982.2024.2403794. Epub 2024 Sep 20. Int J Circumpolar Health. 2024. PMID: 39303209 Free PMC article.
-
Effect of anti-inflammatory diets on health-related quality of life in adults with chronic disease: a systematic review and meta-analysis.BMJ Nutr Prev Health. 2025 Jun 10;8(1):e001257. doi: 10.1136/bmjnph-2025-001257. eCollection 2025. BMJ Nutr Prev Health. 2025. PMID: 40771529 Free PMC article.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
