Evidence for Subcortical Plasticity after Paired Stimulation from a Wearable Device
- PMID: 33441436
- PMCID: PMC7896019
- DOI: 10.1523/JNEUROSCI.1554-20.2020
Evidence for Subcortical Plasticity after Paired Stimulation from a Wearable Device
Abstract
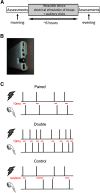
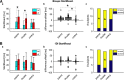
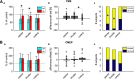
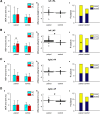
Existing non-invasive stimulation protocols can generate plasticity in the motor cortex and its corticospinal projections; techniques for inducing plasticity in subcortical circuits and alternative descending pathways such as the reticulospinal tract (RST) are less well developed. One possible approach developed by this laboratory pairs electrical muscle stimulation with auditory clicks, using a wearable device to deliver stimuli during normal daily activities. In this study, we applied a variety of electrophysiological assessments to male and female healthy human volunteers during a morning and evening laboratory visit. In the intervening time (∼6 h), subjects wore the stimulation device, receiving three different protocols, in which clicks and stimulation of the biceps muscle were paired at either low or high rate, or delivered at random. Paired stimulation: (1) increased the extent of reaction time shortening by a loud sound (the StartReact effect); (2) decreased the suppression of responses to transcranial magnetic brain stimulation (TMS) following a loud sound; (3) enhanced muscle responses elicited by a TMS coil oriented to induce anterior-posterior (AP) current, but not posterior-anterior (PA) current, in the brain. These measurements have all been suggested to be sensitive to subcortical, possibly reticulospinal, activity. Changes were similar for either of the two paired stimulus rates tested, but absent after unpaired (control) stimulation. Taken together, these results suggest that pairing clicks and muscle stimulation for long periods does indeed induce plasticity in subcortical systems such as the RST.SIGNIFICANCE STATEMENT Subcortical systems such as the reticulospinal tract (RST) are important motor pathways, which can make a significant contribution to functional recovery after cortical damage such as stroke. Here, we measure changes produced after a novel non-invasive stimulation protocol, which uses a wearable device to stimulate for extended periods. We observed changes in electrophysiological measurements consistent with the induction of subcortical plasticity. This protocol may prove an important tool for enhancing motor rehabilitation, in situations where insufficient cortical tissue survives to be a plausible substrate for recovery of function.
Keywords: electrical stimulation; long-term potentiation; reticulospinal; spike timing-dependent plasticity.
Copyright © 2021 the authors.
Figures

References
-
- Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 57:289–300. 10.1111/j.2517-6161.1995.tb02031.x - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
