Role of the Nucleus Basalis as a Key Network Node in Temporal Lobe Epilepsy
- PMID: 33441453
- PMCID: PMC8055321
- DOI: 10.1212/WNL.0000000000011523
Role of the Nucleus Basalis as a Key Network Node in Temporal Lobe Epilepsy
Abstract
Objective: To determine whether the nucleus basalis of Meynert (NBM) may be a key network structure of altered functional connectivity in temporal lobe epilepsy (TLE), we examined fMRI with network-based analyses.
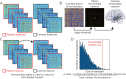
Methods: We acquired resting-state fMRI in 40 adults with TLE and 40 matched healthy control participants. We calculated functional connectivity of NBM and used multiple complementary network-based analyses to explore the importance of NBM in TLE networks without biasing our results by our approach. We compared patients to controls and examined associations of network properties with disease metrics and neurocognitive testing.
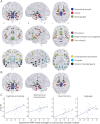
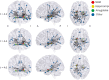
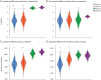
Results: We observed marked decreases in connectivity between NBM and the rest of the brain in patients with TLE (0.91 ± 0.88, mean ± SD) vs controls (1.96 ± 1.13, p < 0.001, t test). Larger decreases in connectivity between NBM and fronto-parietal-insular regions were associated with higher frequency of consciousness-impairing seizures (r = -0.41, p = 0.008, Pearson). A core network of altered nodes in TLE included NBM ipsilateral to the epileptogenic side and bilateral limbic structures. Furthermore, normal community affiliation of ipsilateral NBM was lost in patients, and this structure displayed the most altered clustering coefficient of any node examined (3.46 ± 1.17 in controls vs 2.23 ± 0.93 in patients). Abnormal connectivity between NBM and subcortical arousal community was associated with modest neurocognitive deficits. Finally, a logistic regression model incorporating connectivity properties of ipsilateral NBM successfully distinguished patients from control datasets with moderately high accuracy (78%).
Conclusions: These results suggest that while NBM is rarely studied in epilepsy, it may be one of the most perturbed network nodes in TLE, contributing to widespread neural effects in this disabling disorder.
© 2021 American Academy of Neurology.
Figures

Comment in
-
Are We Inviting Another Attendee to the Temporal Lobe Epilepsy Party?Epilepsy Curr. 2021 Jun 3;21(4):270-272. doi: 10.1177/15357597211021080. eCollection 2021 Jul-Aug. Epilepsy Curr. 2021. PMID: 34690565 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Grants and funding
- R01 NS075270/NS/NINDS NIH HHS/United States
- T32 EB001628/EB/NIBIB NIH HHS/United States
- R01 NS112252/NS/NINDS NIH HHS/United States
- F31 NS106735/NS/NINDS NIH HHS/United States
- T32 GM007347/GM/NIGMS NIH HHS/United States
- R00 NS097618/NS/NINDS NIH HHS/United States
- R01 NS108445/NS/NINDS NIH HHS/United States
- R01 NS095291/NS/NINDS NIH HHS/United States
- T32 EB021937/EB/NIBIB NIH HHS/United States
- R01 NS110130/NS/NINDS NIH HHS/United States
- T32 GM152284/GM/NIGMS NIH HHS/United States
- P50 HD103537/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
