P2RX7 at the Host-Pathogen Interface of Infectious Diseases
- PMID: 33441488
- PMCID: PMC7849353
- DOI: 10.1128/MMBR.00055-20
P2RX7 at the Host-Pathogen Interface of Infectious Diseases
Abstract
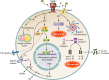
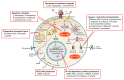
The P2X7 receptor (P2RX7) is an important molecule that functions as a danger sensor, detecting extracellular nucleotides from injured cells and thus signaling an inflammatory program to nearby cells. It is expressed in immune cells and plays important roles in pathogen surveillance and cell-mediated responses to infectious organisms. There is an abundance of literature on the role of P2RX7 in inflammatory diseases and the role of these receptors in host-pathogen interactions. Here, we describe the current knowledge of the role of P2RX7 in the host response to a variety of pathogens, including viruses, bacteria, fungi, protozoa, and helminths. We describe in vitro and in vivo evidence for the critical role these receptors play in mediating and modulating immune responses. Our observations indicate a role for P2X7 signaling in sensing damage-associated molecular patterns released by nearby infected cells to facilitate immunopathology or protection. In this review, we describe how P2RX7 signaling can play critical roles in numerous cells types in response to a diverse array of pathogens in mediating pathogenesis and immunity to infectious agents.
Keywords: P2RX7; infection; inflammasome; inflammatory; purinergic.
Copyright © 2021 American Society for Microbiology.
Figures

Similar articles
-
P2RX7, an adaptive immune response gene, is associated with Parkinson's disease risk and age at onset.J Parkinsons Dis. 2024 Nov;14(8):1575-1583. doi: 10.1177/1877718X241296015. Epub 2024 Dec 1. J Parkinsons Dis. 2024. PMID: 39957192
-
P2RX7 signaling drives the differentiation of Th1 cells through metabolic reprogramming for aerobic glycolysis.Front Immunol. 2023 Mar 13;14:1140426. doi: 10.3389/fimmu.2023.1140426. eCollection 2023. Front Immunol. 2023. PMID: 36993971 Free PMC article.
-
Damage sensing through TLR9 regulates inflammatory and antiviral responses during influenza infection.Mucosal Immunol. 2025 Jun;18(3):537-548. doi: 10.1016/j.mucimm.2025.01.008. Epub 2025 Jan 28. Mucosal Immunol. 2025. PMID: 39884393 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
Cited by
-
The impact of cannabinoids on inflammasome signaling in HIV-1 infection.NeuroImmune Pharm Ther. 2023 Mar 25;2(1):79-88. doi: 10.1515/nipt-2023-0002. Epub 2023 Feb 23. NeuroImmune Pharm Ther. 2023. PMID: 37027347 Free PMC article. Review.
-
Inflammasomes as mediators of inflammation in HIV-1 infection.Transl Res. 2023 Feb;252:1-8. doi: 10.1016/j.trsl.2022.07.008. Epub 2022 Jul 30. Transl Res. 2023. PMID: 35917903 Free PMC article. Review.
-
Both host and parasite non-coding RNAs co-ordinate the regulation of macrophage gene expression to reduce pro-inflammatory immune responses and promote tissue repair pathways during infection with fasciola hepatica.RNA Biol. 2024 Jan;21(1):62-77. doi: 10.1080/15476286.2024.2408706. Epub 2024 Sep 30. RNA Biol. 2024. PMID: 39344634 Free PMC article.
-
Single-Cell Characterization of Calcium Influx and HIV-1 Infection using a Multiparameter Optofluidic Platform.J Vis Exp. 2021 May 18;(171):10.3791/62632. doi: 10.3791/62632. J Vis Exp. 2021. PMID: 34096922 Free PMC article.
-
The Role of Purinergic P2X7 Receptor in Inflammation and Cancer: Novel Molecular Insights and Clinical Applications.Cancers (Basel). 2022 Feb 22;14(5):1116. doi: 10.3390/cancers14051116. Cancers (Basel). 2022. PMID: 35267424 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

