Altered hippocampal gene expression, glial cell population, and neuronal excitability in aminopeptidase P1 deficiency
- PMID: 33441619
- PMCID: PMC7806765
- DOI: 10.1038/s41598-020-79656-6
Altered hippocampal gene expression, glial cell population, and neuronal excitability in aminopeptidase P1 deficiency
Abstract
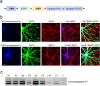
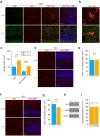
Inborn errors of metabolism are often associated with neurodevelopmental disorders and brain injury. A deficiency of aminopeptidase P1, a proline-specific endopeptidase encoded by the Xpnpep1 gene, causes neurological complications in both humans and mice. In addition, aminopeptidase P1-deficient mice exhibit hippocampal neurodegeneration and impaired hippocampus-dependent learning and memory. However, the molecular and cellular changes associated with hippocampal pathology in aminopeptidase P1 deficiency are unclear. We show here that a deficiency of aminopeptidase P1 modifies the glial population and neuronal excitability in the hippocampus. Microarray and real-time quantitative reverse transcription-polymerase chain reaction analyses identified 14 differentially expressed genes (Casp1, Ccnd1, Myoc, Opalin, Aldh1a2, Aspa, Spp1, Gstm6, Serpinb1a, Pdlim1, Dsp, Tnfaip6, Slc6a20a, Slc22a2) in the Xpnpep1-/- hippocampus. In the hippocampus, aminopeptidase P1-expression signals were mainly detected in neurons. However, deficiency of aminopeptidase P1 resulted in fewer hippocampal astrocytes and increased density of microglia in the hippocampal CA3 area. In addition, Xpnpep1-/- CA3b pyramidal neurons were more excitable than wild-type neurons. These results indicate that insufficient astrocytic neuroprotection and enhanced neuronal excitability may underlie neurodegeneration and hippocampal dysfunction in aminopeptidase P1 deficiency.
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Suppression of exaggerated NMDAR activity by memantine treatment ameliorates neurological and behavioral deficits in aminopeptidase P1-deficient mice.Exp Mol Med. 2022 Aug;54(8):1109-1124. doi: 10.1038/s12276-022-00818-9. Epub 2022 Aug 3. Exp Mol Med. 2022. PMID: 35922532 Free PMC article.
-
Deficiency of aminopeptidase P1 causes behavioral hyperactivity, cognitive deficits, and hippocampal neurodegeneration.Genes Brain Behav. 2018 Feb;17(2):126-138. doi: 10.1111/gbb.12419. Epub 2017 Sep 15. Genes Brain Behav. 2018. PMID: 28834604
-
Hippocampal neurons and glia in epileptic EL mice.J Neurocytol. 2002 Sep-Nov;31(8-9):681-92. doi: 10.1023/a:1025747813463. J Neurocytol. 2002. PMID: 14501207
-
Age-related biophysical alterations of hippocampal pyramidal neurons: implications for learning and memory.Ageing Res Rev. 2002 Apr;1(2):181-207. doi: 10.1016/s1568-1637(01)00009-5. Ageing Res Rev. 2002. PMID: 12039438 Review.
-
Heterogeneity and function of hippocampal macroglia.Cell Tissue Res. 2018 Sep;373(3):653-670. doi: 10.1007/s00441-017-2746-1. Epub 2017 Dec 4. Cell Tissue Res. 2018. PMID: 29204745 Review.
Cited by
-
Systematic Review and Meta-Analyses of Aminopeptidases as Prognostic Biomarkers in Amyotrophic Lateral Sclerosis.Int J Mol Sci. 2023 Apr 12;24(8):7169. doi: 10.3390/ijms24087169. Int J Mol Sci. 2023. PMID: 37108335 Free PMC article.
-
Suppression of exaggerated NMDAR activity by memantine treatment ameliorates neurological and behavioral deficits in aminopeptidase P1-deficient mice.Exp Mol Med. 2022 Aug;54(8):1109-1124. doi: 10.1038/s12276-022-00818-9. Epub 2022 Aug 3. Exp Mol Med. 2022. PMID: 35922532 Free PMC article.
-
The phosphorylation status of eukaryotic elongation factor-2 indicates neural activity in the brain.Mol Brain. 2021 Sep 15;14(1):142. doi: 10.1186/s13041-021-00852-0. Mol Brain. 2021. PMID: 34526091 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

